
Under the lens:
and the Impact of Fenofibrate
Progression of Diabetic Retinopathy
Open Meeting Summary

Meeting Summary
With an increasing prevalence of diabetic retinopathy, a cause of vision impairment and blindness in those with diabetes, there is a need for early detection and timely intervention. This article considers the burden of diabetic retinopathy and the impact of fenofibrate, a peroxisome proliferator-activated receptor-α (PPAR-α) agonist, in reducing the progression of diabetic retinopathy.




Cardiology
Diabetes

This interactive �article was funded by Abbott & intended for HCPs in countries with indication approval
Author:


Hannah Moir1,2 �1. EMJ, London, UK; 2. School of Life Sciences, Pharmacy and Chemistry, Faculty of Health, Science, Social Care and Education, Kingston University, London, UK

Article Title:
Under the LENS: Progression of Diabetic Retinopathy and the Impact of Fenofibrate
Author:
Hannah Moir1,2
1. EMJ, London, UK
2. School of Life Sciences, Pharmacy and Chemistry, Faculty of Health, Science, Social Care and Education, Kingston University, London, UK
Acknowledgements:
The author is a Senior Medical Writer at EMJ, London, UK, and Senior Fellow of Kingston University London, UK.
Disclaimer:
Not all medicines and/or indications presented in this report may be approved for use in certain regions or countries. Please consult your local prescribing information. This content is intended for healthcare professionals from Argentina, Central American cluster, Ecuador, Egypt, Iraq, Malaysia, Mexico, Peru, Philippines, Singapore, Thailand, Turkey, UAE, Ukraine, Uzbekistan, and Vietnam only.
Keywords:
Diabetes, diabetic retinopathy, fenofibrate, maculopathy, macular oedema.
Support statement:
The publication of this interactive article was funded by Abbott.

INTRODUCTORY QUIZ:

2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?

1. Globally, approximately how many people currently have diabetic retinopathy?



3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?


4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?


1. Globally, approximately how many people currently have diabetic retinopathy?

1. Globally, approximately how many people currently have diabetic retinopathy?

A. 29 million

A. 29 million

B. 45 million

B. 45 million

C. 103 million

C. 103 million

D. 160 million

D. 160 million

A. 29 million

B. 45 million

C. 103 million

D. 160 million
2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?


A. Dilated fundus photography

A. Dilated fundus photography

B. Direct ophthalmoscopy

B. Direct ophthalmoscopy

C. Indirect ophthalmoscopy

C. Indirect ophthalmoscopy

D. Teleophthalmology

D. Teleophthalmology

A. Dilated fundus photography

B. Direct ophthalmoscopy

C. Indirect ophthalmoscopy

D. Teleophthalmolog
2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?

3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?
A. Activates peroxisome proliferator-activated receptor-α (PPARα)
B. Activates nuclear factor erythroid 2-related factor (NRF2)
C. Inhibits protein �kinase C (PKC)
D. Inhibits vascular endothelial growth factor(VEGF)
3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?

D. Inhibits vascular endothelial growth factor(VEGF)
A. Activates peroxisome proliferator-activated receptor-α (PPARα)
B. Activates nuclear factor erythroid 2-related factor (NRF2)
C. Inhibits protein �kinase C (PKC)
A. Activates peroxisome proliferator-activated receptor-α (PPARα)
B. Activates nuclear factor erythroid 2-related factor (NRF2)
C. Inhibits protein �kinase C (PKC)
D. Inhibits vascular endothelial growth factor(VEGF)

4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?

4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?

A. 23%

A. 23%

B. 27%


C. 29%

C. 29%

D. 31%

D. 31%

A. 23%

B. 27%

C. 29%

D. 31%
B. 27%






THE INCREASING BURDEN OF DIABETIC RETINOPATHY






Diabetic retinopathy is a common microvascular complication associated with diabetes mellitus (Figure 1).1,2
The increase in the prevalence of diabetic retinopathy will likely continue to strain healthcare systems & resources, including screening, diagnosis, and management, with a substantial associated economic cost.4
However, this can be avoided with early detection and timely intervention. Preventing occurrence, and if present, slowing, or preventing progression must be pursued to save sight.6
There are few effective options to slow the progression of diabetic retinopathy.12 Therapies that are beneficial earlier in the disease progression and that are more cost-effective are needed to reduce the chances of people with diabetes developing progressive diabetic retinopathy.10,12






THE INCREASING BURDEN OF DIABETIC RETINOPATHY
If diabetic retinopathy remains undetected, undiagnosed, or untreated, it can progress to referrable diabetic retinopathy, such as proliferative diabetic retinopathy where either or both retinal neovascularisation and vitreous haemorrhage can be observed, leading to retinal detachment and neovascular glaucoma, resulting in visual impairment.

Figure 1: Pathophysiology of diabetic retinopathy
Proliferative diabetic retinopathy can also cause diabetic maculopathy with exudates beyond the microaneurysms, and/or haemorrhages one observes in non-proliferative diabetic retinopathy, which may progress to macular oedema and threaten vision.7,8



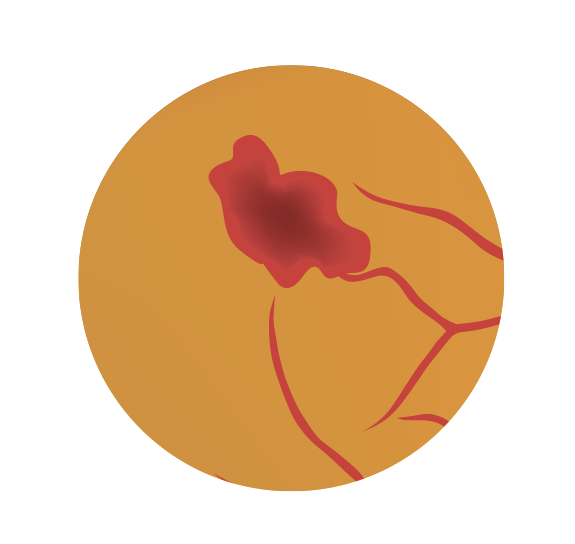
Haemorrhages
“Cotton wool” spots


Newly formed blood vessels (neovascularisation)



Hard exudates




Diabetic retinopathy is a leading cause of blindness, and its prevalence is increasing in many countries.1
With a projected increase in the number of people living with diabetes, along with an ageing global population, and those with diabetes living longer, the number of cases of diabetic retinopathy and resultant visual impairment is expected to rapidly rise.1,2
One study estimated that globally, approximately 103.1 million people may have diabetic retinopathy, which is estimated to increase to 160.5 million by 2045.
In addition, 28.5 million people may have �sight‐threatening stages of diabetic retinopathy, which is estimated to increase to 44.8 million by 2045.2 Globally, approximately 1.07 million people have lost their sight to diabetic retinopathy and nearly 3.28 million are visually impaired.3
Prevalence


• R0 - No apparent lesions
�• R1 - Background retinopathy (mild-
to-moderate non-proliferative
diabetic retinopathy [NDPR])
�• R2 - Pre-proliferative retinopathy� (moderate-to-severe NDPR)
�• R3 - Proliferative retinopathy � / severe










Diabetic retinopathy is a significant public health burden, with social and economic implications for the individual and the community and an impact on economic productivity.4
The global annual economic burden of diabetic retinopathy is estimated to range from, for example, 0.5 billion USD in the USA to 6.4 billion USD in the Caribbean region.3,5
Burden





$0.5b
$6.4b




• For those with diabetes, regular diabetic eye screening (DES), or diabetic retinal screening, is recommended to identify diabetic retinopathy with frequency being dependent on severity.7,9
• This may include the use of direct or indirect ophthalmoscopy, slit-lamp biomicroscopy, and/or optical coherence tomography to detect macular oedema, as well as mydriatic and nonmydriatic retinal fundus photography, which is considered the gold-standard.8,9
1. Diagnosing Diabetic Retinopathy









The management and treatment of diabetic retinopathy involve several options:7
• Eye Surgery:
- Retinal laser therapy - laser photocoagulation
- Vitrectomy surgery
• Intravitreal eye injections with anti-vascular endothelial growth factor (anti-VEGF) for the management of diabetic macular oedema
• Corticosteroids
• Lifestyle and systemic management strategies: such as maintaining a healthy diet, physical activity, and controlling cholesterol and blood pressure levels.
- Many of these treatments, particularly for sight-threatening diseases, such as retinal laser and intravitreal injections, require expertise to deliver, are invasive, and can involve significant time and expense for both the individual and the healthcare system.
- Although sight‐saving in many cases, these therapeutic modalities have inherent risks, and visual loss can still occur in a proportion of people who have little to no response to the treatments.10,11
Managing Diabetic Retinopathy







TAKING A GLANCE AT FENOFIBRATE
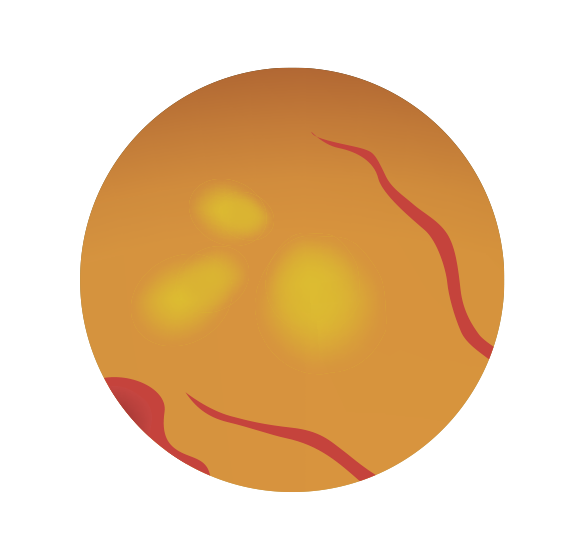
Fenofibrate, a third-generation fibric acid derivative, is a peroxisome proliferator-activated �receptor-α (PPAR-α) agonist indicated for reducing circulating low-density lipoprotein- (LDL-) cholesterol, and triglycerides, and increase high-density lipoprotein- (HDL-) cholesterol (Figure 2).7,13


First Look at Fenofibrate for Diabetic Retinopathy
It has now been observed that fenofibrate may be a useful strategy to slow the development and progression of diabetic retinopathy, particularly Type 2 diabetes with pre-existing retinopathy.6
The potential role of fenofibrate in diabetic retinopathy was initially highlighted in the subsidiary clinical outcomes from landmark randomised controlled trials, such as the FIELD, ACCORD, and ECLIPSE-REAL studies, examining the impact of fenofibrate on cardiovascular risk in those
with diabetes.7,14-17
However, until recently, there have been no large-scale trials confirming the role of fenofibrate in diabetic retinopathy.12








In the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study, conducted in adults with Type 2 diabetes, fenofibrate showed a 31% reduction in patients with proliferative retinopathy or macular oedema needing first laser treatment over a 5-year period.14,15


• The largest multi-national randomised interventional study to date on patients with Type 2 diabetes aged 50–75 years old (N=9,795) compared 200 mg daily fenofibrate monotherapy (n=4,895) with matching placebo (n=4,900).14,15
• The primary outcomes of the study demonstrated that patients with Type 2 diabetes who took fenofibrate for 5 years (in addition to therapies for hyperglycaemia and other risk factors for retinopathy) had significant reductions in total cardiovascular disease events, particularly nonfatal myocardial infarction (MI) and coronary revascularisation.14,15,17


Field
31
%




• Fewer patients in the fenofibrate group (3.1%) had a 2-step progression than those in the placebo group (14.6%; p<0.05).15
• Fewer patients in the fenofibrate group have a two-step progression of retinopathy grade, macular oedema, or laser treatments than in the placebo group (HR: 0.66; 95% CI: 0.47–0.94; p<0.05).15
• In the tertiary outcome, there was a 31% proportional reduction in the need for first retinal laser therapy for diabetic retinopathy (either macular oedema or proliferative retinopathy) in the fenofibrate group over 5 years.14,15
• There was a relative reduction in the fenofibrate group (3.4%) compared with the placebo group (4.9%; hazard ratio [HR]: 0.69; 95% CI: 0.56–0.84; p<0.001).
• Sub-study analysis indicated progression of retinopathy grade did not differ between the two groups nor in patients without pre-existing retinopathy.15

Field




Figure 2: Classic Mechanism of Action Pathway of Fenofibrate13 �Fenofibrate activates PPAR-α, which lowers free fatty acids by upregulating the synthesis of proteins responsible for fatty acid transport and ß-oxidation. These proteins also include lipoprotein lipase, and apolipoproteins A-I and A-II. As a result, this action inhibits the formation of triglycerides and low-density lipoproteins (LDL) and increases high-density lipoproteins (HDL). Additionally, it downregulates apolipoprotein C-III (Apo-CIII), a key regulator of atherosclerosis.13




• A randomised trial was conducted in the USA and Canada. Participants with Type 2 diabetes at high risk for cardiovascular disease (N=10,251) were assigned to receive simvastatin in combination with either 160 mg daily fenofibrate or placebo (in addition to intensive glycaemic control, and/or blood pressure control, or standard therapy).16
• The study demonstrated fenofibrate in combination with statin, slowed the progression of diabetic retinopathy in patients with Type 2 diabetes.
• The rate of progression of diabetic retinopathy at 4 years was 6.5% in the fenofibrate group and 10.2% in the placebo group (adjusted odds ratio: 0.60; 95% CI: 0.42–0.87; p<0.05).16
40
%
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) and ACCORD Eye studies further supported the suggestion that fenofibrate may reduce the progression of diabetic retinopathy in adults with Type 2 diabetes by 40%.7,15-17

Accord




• The rate of moderate vision loss was 16.0% in the fenofibrate group compared with 15.2% in the placebo group (adjusted HR: 1.04; 95% CI: 0.83–1.32; p>0.05).16
• A sub-group of eligible patients (N=2,856) were enrolled into the ACCORD-Eye study, which consisted of two standardised eye examinations along with fundus photographs at baseline and year 4 follow-up.16
• A sub-group of these participants were also enrolled into the ACCORD Lipid study which reported a 40% proportional reduction in the composite outcome of three-step ETDRS diabetic retinopathy progression, retinal laser, or vitrectomy over 4 years in patients treated with fenofibrate and statin combination therapy compared to statin therapy alone.16






Accord


Diabetic retinopathy�examined by�fundus photography




• A propensity-matched cohort study conducted in Korea. Patients with Type 2 diabetes and metabolic syndrome aged ≥30 years old (N=1,117,422) were assigned to receive statin therapy in combination with fenofibrate (n=232,091) or statin alone (n=885,331).18
• Composite of diabetic retinopathy progression (including vitreous haemorrhage, vitrectomy, laser photocoagulation, intra-vitreous injection therapy, and retinal detachment) was measured at follow-up of 4 years.
16
%
The ECLIPSE-REAL (Effectiveness of Fenofibrate Therapy in Residual Cardiovascular Risk Reduction in the Real World Setting) study compared the cardiovascular events in Korean patients with metabolic syndrome (aged 30 years and above) who were given statin monotherapy with those given combination therapy (fenofibrate and statin). The study demonstrated a 16% reduction in the risk of diabetic retinopathy progression in the latter group.18,19


ECLIPSE-REAL




The ECLIPSE-REAL Study
• The addition of fenofibrate in combination with statins was associated with a lower risk of diabetic retinopathy progression in patients with Type 2 diabetes and metabolic syndrome.
• The statin plus fenofibrate group was associated with a 14% lower risk of vitreous haemorrhage, 14% lower rates of laser photocoagulation, and 27% lower rates of intra-vitreous injection therapy compared with the statin-only group (p<0.05).
• Patients with pre-existing retinopathy at baseline showed notable benefits from fenofibrate treatment (HR: 0.83; 95% CI: 0.73–0.95; p<0.05).18,19




ECLIPSE-REAL



PEERING THROUGH THE LENS OF FENOFIBRATE FOR DIABETIC RETINOPATHY
To confirm these findings, clinical trials have since been conducted to investigate the ocular effects of fenofibrate (Figure 3) in those with diabetes.7,17





The LENS (Lowering Events in Non-proliferative Retinopathy in Scotland) trial is the first large-scale, Phase III randomised, parallel-group, double-masked, placebo-controlled clinical trial conducted within a national multi-centre retinal screening, specifically designed to investigate the effect of fenofibrate on the progression of retinopathy in people with early diabetic eye disease.12
Participants: �The study recruited 1,151 with non-referable diabetic retinopathy or maculopathy,�identified through the National DES programme at 16 sites across Scotland, UK.12
Trial Design:�Participants were randomly assigned to receive either 145 mg fenofibrate tablets or placebo (taken daily or, in those with impaired renal function, on alternate days). The follow-up was median 4 years (interquartile range: 3.6–4.3 years) with adherence 88–89% (Figure 4).7,12










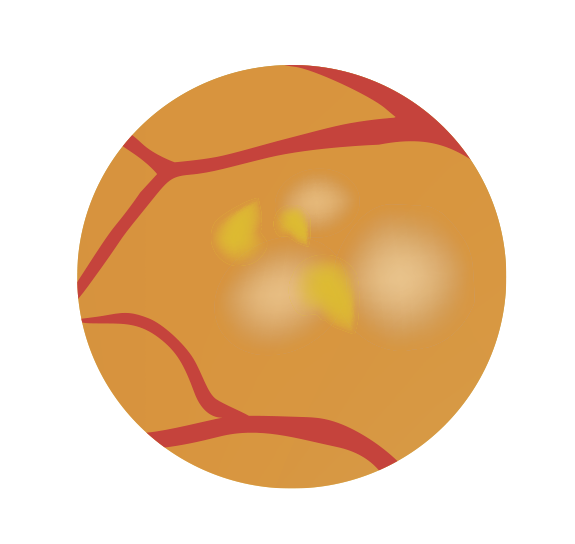
Fenofibrate is hypothesised to slow the progression of diabetic retinopathy by reducing inflammation in the retina, as well as improving dyslipidaemia and hypertriglyceridaemia.6 There is growing evidence to indicate that fenofibrate directly affects the eye by reducing retinal vascular leakage, and retinal inflammation, and potentially play a protective role in maintaining the blood–retina barrier.21-23

Figure 3: Mechanism of Action of Fenofibrate in Reducing the Progression of Diabetic Retinopathy.20








Endothelial cell loss
Neovessels




Fenofibric acid



Pericyte
Inflammatory cells (macrophages) NF-kB, IL-6, TNF +VEGF
Microglial




The mechanism of action of fenofibrate in reducing diabetic retinopathy progression is thought to be more complex than the lipid-lowering effects indicated for the cardiovascular effects. Several mechanisms have been proposed including:
• Increasing circulating apolipoprotein A-I, an independent protective factor for diabetic retinopathy.24
• Intra-retinal lipid metabolism and reducing lipid deposition and lipotoxicity25
• Anti-apoptotic, anti-oxidant, anti-inflammatory and anti-angiogenic activity, and protective effects on the blood-retinal barrier breakdown.23
• Inhibit cytokine-induced NF-kB activity, as well as monocyte chemoattractant-1 and intracellular adhesion molecule-1.
• Increased expression of vascular endothelial growth factor receptor 3 and matrix metallopeptidase,13 and corneal neovascularisation formation through PPARα downregulation.26
• Reduced TNF-α and IL-6 in the cornea, leading to the suppression of inflammation.
Continued...





Figure 4: Clinical Trial Design of LENS






















Adults aged 18 years and older with diabetes mellitus and non-referable (early) moderately severe diabetic retinopathy or maculopathy located across mainland Scotland.
• Nonreferable disease, defined as mild background retinopathy in both eyes or observable background retinopathy in one/both eyes at the most recent retinal screening assessment or observable maculopathy in one/both eyes at a retinal screening assessment in the past 3 years (though participants were invited based on nonreferable disease at their most recent retinal screening).
• Estimated glomerular filtration rate (eGFR) ≥40 mL/min/1.73 m2 90% (n=518) fenofibrate group had eGFR ≥ 60 mL/min/1.73 m2 93% (n=535) placebo group had eGFR ≥ 60 mL/min/1.73 m2
* Blot haemorrhage has the same or greater diameter as a retinal vein crossing the optic disc † R4i is not counted towards the primary outcome as it represents inactive disease
‡ At the start of LENS in 2018 and until end-2021, all patients in Scotland with M2 retinal screening results required referral to a specialist in ophthalmology. During 2022, the Diabetic Eye Screening programme started to introduce a phased change to the management pathway of patients with M2 disease. In the new pathway, M2 in the context of poor visual acuity (e.g., 6/9.5 or worse) leads to optical coherence tomography (OCT) imaging followed by referral to an ophthalmologist if there is evidence of macular oedema.
Pre-Screening Eligibility Criteria:
Click for: Table. NHS Scotland’s Diabetic Eye Screening Programme Grading Scheme for Retinopathy and Maculopathy





10-week open-label active pre-randomisation run-in with 145 mg nanoparticle fenofibrate tablets*
• At ~8 weeks, participants attended an in-person randomisation assessment.
• eGFR ≥30 mL/min/1.73 m2 (lower than the threshold at the screening assessment) to allow for the anticipated increase in serum creatinine due to fenofibrate treatment during the run-in.
*≥eGFR of 60 L/min/ 1.73 m2 one tablet daily, �eGFR of 40–59 mL/min/ 1.73 m2 one tablet on alternate days
Pre-randomisation:

Participants were randomised to 145 mg nanoparticle fenofibrate tablets or matching placebo in a 1:1 ratio *dose frequency depended on renal function.
• 576 allocated fenofibrate and follow-up (completed)
• 575 allocated placebo and follow-up, 573 completed. 1 consent withdrawn, 1 lost to follow-up.
Randomised Clinical Trial Design:
*eGFR of ≥60 mL/min/1.73 m2 one tablet daily�eGFR of 30–59 mL/min/1.73 m2 one tablet on alternate days
A 14% increase in those with eGFR<60 mL/min/ 1.73m2 was attributed to the effect of fenofibrate.


DES retinal screening was performed, and participants were contacted every 6 months through telephone follow-up
• Option: Follow-up through medical record review or contact with their usual doctor if they were not contactable or declined further contact.
Follow-up:
eGFR results were monitored centrally from routine care via electronic patient records
• If post-randomisation eGFR fell to 30–59 mL/min/1.73 m2, trial treatment was reduced to one tablet on alternate days
• If below 30 mL/min/1.73 m2, trial treatment was discontinued�but could be recommenced if renal function subsequently improved.

• If post-randomisation eGFR fell to 30-59ml/min/1.73 m2, trial treatment was reduced to one tablet on alternate days,
• If below 30ml/min/1.73 m2, trial treatment was discontinued � but could be recommenced if renal function subsequently improved.



LENS Clinical Trial Endpoints



PRIMARY
SECONDARY
SAFETY



Main outcomes:
The study demonstrated that those who received fenofibrate had a 27% reduced risk of diabetic retinopathy progression compared to placebo, as indicated by a lower risk of needing specialist care or treatment for diabetic retinopathy or maculopathy over 4 years.7 The study also demonstrated fenofibrate was associated with a lower risk of developing macular oedema.7

27
%
PRIMARY ENDPOINTS
PRIMARY RESULTS
PRIMARY RESULTS
PRIMARY OUTCOMES
Figure 5: Occurrence of Primary Outcome Events (*Referable Diabetic Retinopathy or Maculopathy, or Treatment for Diabetic Retinopathy or Maculopathy).⁷

• Composite of developing referable diabetic retinopathy or treatment (intravitreal injection, retinal laser, vitrectomy) for retinopathy or maculopathy.
• Time to the first occurrence of the composite of developing referable diabetic retinopathy or maculopathy, or treatment for diabetic retinopathy or maculopathy (including intravitreal injection of medication, retinal laser therapy, or vitrectomy) in either eye.7
• Referable diabetic retinopathy or maculopathy was defined according to NHS Scotland’s DES Programme Grading Scheme as referable background (i.e., moderately severe or severe non-proliferative) diabetic retinopathy, or proliferative diabetic retinopathy, or referable maculopathy (any blot haemorrhage or exudate within one disc diameter distance of the foveal centre).

Progression from non-referable to referable eye disease in the target population would occur in approximately 29% of individuals over 4 years.12
29
%
Of 1,151 participants, progression to referable diabetic retinopathy or maculopathy, or treatment thereof occurred in 22.7% in the fenofibrate group (n=131) compared to 29.2% in the placebo group (n=168) (HR: 0.73; 95% CI: 0.58-0.91; P<0.05) over a median of 4 years, representing an absolute reduction of 6.5% (95% CI: 1.4–11.5%; FIgure 5).7





SECONDARY ENDPOINTS
SECONDARY RESULTS
Time to any progression of diabetic retinopathy or maculopathy; the development of referable maculopathy alone; the development of macular oedema (including centre-involving and non-centre-involving macular oedema as identified during slit lamp examination or optical coherence tomography); change in visual function (based on Visual Function Questionnaire-25 data), quality of life (based on EQ-5D-5L questionnaire data) and visual acuity; components of the primary outcome (namely, development of referable diabetic retinopathy or maculopathy, and treatment thereof); and the primary outcome in six prespecified subgroups, namely men versus women, age <60 versus ≥60 years, Type 1 diabetes versus Type 2 diabetes and other types, randomisation eGFR <60 versus ≥60 mL/min/1.73 m2, HbA1c <70 mmol/mol versus ≥70 mmol/mol, and the timing of the primary outcome (first year after randomisation versus later years).7

SECONDARY ENDPOINTS
SECONDARY RESULTS
• Treatment for diabetic retinopathy or maculopathy occurred in 3% of the fenofibrate group and 4.9% of the placebo group (HR:0.58; 95% CI:0.31–1.06; figure 6).7
• The frequency for any progression of retinopathy or maculopathy occurred in 32.1% in the fenofibrate group compared to 40.2% in the placebo group (HR: 0.74; 95% CI: 0.61–0.90).7
• A similar frequency for any progression of maculopathy occurred in 18.6% in the fenofibrate group compared with 25.9% in the placebo group (HR: 0.66; 95% CI: 0.52–0.85).
• The development of macular oedema occurred in 3.8% in the fenofibrate group compared to 7.5% in the placebo group (HR: 0.50; 95% CI: 0.30–0.84).7
• Seventeen participants (3.0%) in the fenofibrate group were given treatment for retinopathy compared with 28 participants (4.9%) in the placebo group (HR: 0.58; 95% CI: 0.31–1.06).7
• There was no effect on visual function, quality of life, or visual acuity.





Primary Outcome Events Referable Diabetic
Retinopathy or Maculopathy
Treatment For Diabetic Retinopathy or Maculopathy
Primary Composite Outcome*
Secondary Eye Outcome Events
Any Progression of Diabetic Retinopathy or Maculopathy
Development of Exudates or Blot Haemorrhages Within One Disc Diameter of The Macula
Development of Macular Oedema
Primary Outcome Events Referable Diabetic
Retinopathy or Maculopathy Treatment For Diabetic Retinopathy or Maculopathy
Figure 6: Hazard Ratios (95% CI) of Primary Composite and Secondary Eye Outcomes.7
SECONDARY results
Sub-group analysis
Figure 7: Hazard Ratios (95% CI) For The Primary Outcome in Pre-specified Subgroups Defined According to Baseline Characteristics.7
There was no differential proportional effects within prespecified subgroup categories, including Type 1 versus Type 2 and other types of diabetes and normal versus impaired renal function.7


Sub-group analysis
SUB-GROUP ANALYSIS
SECONDARY endpoints
SAFETY Outcomes
• There was no difference in the occurrence of major cardiovascular events in the fenofibrate group (7.8%) compared with the placebo group (7.5%) (HR: 1.05; 95 CI: 0.69–1.60) or non-traumatic lower limb amputation in the fenofibrate group (0.7%) compared with the placebo group (1.9%) (HRn: 0.35; 95% CI: 0.11–1.12) and no effect on urine albumin: creatinine ratio in the fenofibrate group (13.6; 95% CI: 12.1–15.3) compared with the placebo group (15.5; 95% CI: 13.8–17.5) with mean difference between groups of -12.4% (95% CI: -25.8%– -3.5%).7
• Overall, 35 (6.1%) participants in the fenofibrate group and 38 (6.6%) participants in the placebo group died.7
• Serious adverse events occurred in 208 (36.1%) participants in the fenofibrate group and 204 (35.5%) participants in the placebo group.7
• Adverse event reports of surgical and medical eye procedures, vitreous haemorrhages, and macular oedema were adjudicated by doctors at the CCO, masked to treatment allocation.7

KEY TAKEAWAYS
• Fenofibrate showed a reduction in the relative rate of progression of diabetic retinopathy by 27% when compared with placebo in those with early diabetic retinal changes.7
• Fenofibrate is a cost-effective option in managing diabetic retinopathy.29
• Treatment with fenofibrate appeared similarly effective in those with Type 2 diabetes to that seen in subsidiary analyses from the cardiovascular trials.14-18
FUTURE INSIGHTS:
The potential impact of fenofibrate on diabetic retinopathy is anticipated to be further elucidated in the upcoming years through several key clinical trials :






FAME-EYE�The Fenofibrate And Microvascular�Events in Type 1 Diabetes Eye �(FAME-1 EYE).27
TREATMENT PARADIGM�Fenofibrate for Prevention of DR�Worsening (Protocol AF).28



The Fenofibrate And Microvascular Events in Type 1 Diabetes Eye (FAME-1 EYE).27
• A Phase III randomised multicentre double-blind, placebo-controlled study taking place in Australia and New Zealand, and internationally (including Hong Kong, Northern Ireland, and the UK).
• Evaluating the efficacy and safety of 145 mg fenofibrate or placebo once daily in adults with Type 1 diabetes mellitus ≥18 years old and pre-existing non-proliferative diabetic retinopathy.
• Primary outcome will measure central zone macular thickness and total macular volume measured using optical coherence tomography at 12 months and followed up 3–6 monthly for an average of 3 years.
• Recruitment includes at least 450 participants, both men and non-pregnant women aged 18 years or over, adults with Type 1 diabetes and non-proliferative diabetic retinopathy (NPDR) with an Early Treatment Diabetic Retinopathy Study (ETDRS) score of 35–53.
• Recruitment started in 2016, with primary completion estimated for 2026.


• A Phase III randomised, placebo-controlled study taking place in the USA through the Diabetic Retinopathy Clinical Research Retina Network is now conducting a controlled clinical trial of fenofibrate. The randomised trial will evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening through 6 years of follow-up in eyes with mild-to-moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
• Evaluating the efficacy of either 160 mg or 54 mg fenofibrate based on eGFR value at screening, or placebo, once daily in at least 560 participants aged ≥18–80 years with Type 1 or Type 2 diabetes, with mild-to-moderately severe non-proliferative diabetic retinopathy.
• Primary outcome will measure worsening of diabetic retinopathy over a 6-year follow-up with the development of centre-involved diabetic macular oedema with visual acuity loss through 4 years.
• The study will also evaluate the feasibility of ophthalmologists in prescribing fenofibrate (with continued communication and monitoring from the primary care physician), to determine whether fenofibrate reduces the risk of diabetic retinopathy progression using this treatment paradigm.
• Primary completion is estimated for 2029.
TREATMENT PARADIGM - Fenofibrate for Prevention of DR Worsening (Protocol AF).28




1. Globally, approximately how many people currently have diabetic retinopathy?


1. Globally, approximately how many people currently have diabetic retinopathy?

1. Globally, approximately how many people currently have diabetic retinopathy?

A - 29 million

A - 29 million

B - 45 million

B - 45 million

C - 103 million

C - 103 million

D - 160 million

D - 160 million

A - 29 million

B - 45 million

C - 103 million

D - 160 million


2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?

2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?

2. Which diagnostic tool is recommended as the gold standard to identify �diabetic retinopathy?

A. Dilated fundus photography

A. Dilated fundus photography

B. Direct ophthalmoscopy

B. Direct ophthalmoscopy

C. Indirect ophthalmoscopy

C. Indirect ophthalmoscopy

D. Teleophthalmology

D. Teleophthalmology

B. Direct ophthalmoscopy

C. Indirect ophthalmoscopy

A. Dilated fundus photography

D. Teleophthalmolog


3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?


3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?

3. What is the proposed mechanism of action of fenofibrate on cardiovascular effects in diabetes?
A - Activates peroxisome proliferator-activated receptor-α (PPARα)
A - Activates peroxisome proliferator-activated receptor-α (PPARα)
B - Activates nuclear factor erythroid 2-related factor (NRF2)
B - Activates nuclear factor erythroid 2-related factor (NRF2)
C - Inhibits protein �kinase C (PKC)
C - Inhibits protein �kinase C (PKC)
D - Inhibits vascular endothelial growth factor(VEGF)
D - Inhibits vascular endothelial growth factor(VEGF)
A - Activates peroxisome proliferator-activated receptor-α (PPARα)
B - Activates nuclear factor erythroid 2-related factor (NRF2)
C - Inhibits protein �kinase C (PKC)
D - Inhibits vascular endothelial growth factor(VEGF)



4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?


4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?

4. Findings from the LENS study reported that fenofibrate reduced the relative progression rate of diabetic retinopathy by how much?

A - 23%

A - 23%

B - 27%

B - 27%

C - 29%

C - 29%

D - 31%

D - 31%

C - 29%

A - 23%

B - 27%

D - 31%

POST ARTICLE QUIZ:


5. How valuable did you find this article in enhancing your understanding of diabetic retinopathy and its management options?


5. How valuable did you find this article in enhancing your understanding of diabetic retinopathy and its management options?

A. Extremely valuable – substantial new insights and knowledge
C. Slightly valuable – a few new points of interest

5. How valuable did you find this article in enhancing your understanding of diabetic retinopathy and its management options?
A. Extremely valuable - substantial new insights and knowledge
B. Moderately valuable – some useful information
B. Moderately valuable - some useful information
C. Slightly valuable – a few new points of interest
D. Not valuable – no new or �useful information
D. Not valuable – no new or �useful information
A. Extremely valuable - substantial new insights and knowledge
B. Moderately valuable - some useful information
C. Slightly valuable – a few new points of interest
D. Not valuable – no new or �useful information

6. Is there anything else you would like to learn about diabetic retinopathy or its management that was not covered in this article?


6. Is there anything else you would like to learn about diabetic retinopathy or its management that was not covered in this article?

A. Yes – I would like to learn �more on advances in pharmacological treatments
B. Yes – I would to learn more on long-term outcomes
C. Yes – I would like to learn more on diagnostic approaches for earlier detection
D. No – the article covered everything I wanted to know
D. Not valuable – no new or �useful information

6. Is there anything else you would like to learn about diabetic retinopathy or its management that was not covered in this article?
A. Yes – I would like to learn �more on advances in pharmacological treatments
B. Yes – I would to learn more on long-term outcomes
C. Yes – I would like to learn more on diagnostic approaches for earlier detection
D. No – the article covered everything I wanted to know


A. Yes – I would like to learn �more on advances in pharmacological treatments
B. Yes – I would to learn more on long-term outcomes
C. Yes – I would like to learn more on diagnostic approaches for earlier detection


GLO2332127


21. Chen Y et al. Therapeutic effects of PPARa agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62(1):261-72.
22. Fu D et al. Effects of modified low-density lipoproteins and fenofibrate on an outer blood–retina barrier model: implications for diabetic retinopathy. J Ocul Pharmacol Ther. 2020;36(10):754-64.
23. Wong TY et al. Fenofibrate - a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol. 2012;154(1):6-12.
24. Sasongko MB et al. Serum apolipoproteins AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care. 2011;34(2):474-79. 25. Simó R, Hernández C. Fenofibrate for diabetic retinopathy. Lancet. 2007;370(9600):1667-68. 26. Zhao J et al. Fenofibrate inhibits the expression of VEGFC and VEGFR-3 in retinal pigmental epithelial cells exposed to hypoxia. Exp. Med. 2015;10(4):1404-12.
27. University of Sydney. The fenofibrate and microvascular events in type 1 diabetes eye. (FAME 1 EYE). NCT01320345. https://clinicaltrials.gov/study/NCT01320345.
28. Jaeb Center for Health Research. Fenofibrate for prevention of DR worsening (protocol AF). NCT04661358. https://clinicaltrials.gov/study/NCT04661358.
29. Valentine WJ et al. Evaluating the cost-utility of fenofibrate treatment of diabetic retinopathy in Australia. Value Health. 2013;16(7):PA442.�
References cont...



10. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne). 2023;13:1077669.
11. Wells JA et al. Aflibercept, bevacizumab,or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123(6):1351-9.
12. LENS Collaborative Group. Design, recruitment and baseline characteristics of the LENS trial. Diabet Med. 2024;41(9):e15310. doi:10.1111/dme.15310.
13. Staels B et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088-93.
14. Keech A et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-61.
15. Keech AC et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687-97.
16. ACCORD Study Group; ACCORD Eye Study Group; Chew EY et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-44.
17. Preiss D et al. Effect of fenofibrate therapy on laser treatment for diabetic retinopathy: a meta-analysis of randomized controlled trials. Diabetes Care. 2022;45(1):e1-e2.
18. Kim NH et al. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ. 2019;366:l5125.
19. Kim NH et al. Addition of fenofibrate to statins is associated with risk reduction of diabetic retinopathy progression in patients with type 2 diabetes and metabolic syndrome: a propensity-matched cohort study. Diabetes Metab. 2023;49(3):101428.
20. Kusuhara S et al. Pathophysiology of diabetic retinopathy: The old and the new. Diabetes Metab J. 2018;42(5):364-76.
References cont...



1. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-e160.
2. Teo ZL et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580-91.
3. Vision Loss Expert Group of the Global Burden of Disease Study; GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 2000 to 2020. Eye (Lond). 2024;38(11):2047-57.
4. World Health Organization (WHO). World report on vision. 2019. Available at: https://www.who.int/publications/i/item/world-report-on-vision. Last accessed: 5 September 2024.
5. Rein DB et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754-60.
6. Kataoka SY et al. Fenofibrate for diabetic retinopathy. Cochrane Database Syst Rev. 2023;6(6):CD013318.
7. Preiss D et al. Effect of fenofibrate on progression of diabetic retinopathy. NEJM Evid. 2024;3(8):EVIDoa2400179.
8. Wong TY et al. Guidelines on diabetic eye care: The International Council of Ophthalmology Recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608-22.
9. WHO Regional Office for Europe. Diabetic retinopathy screening: a short guide. Increase effectiveness, maximize benefits and minimize harm. 2021. Available at: https://www.who.int/europe/publications/i/item/9789289055321. Last accessed: 5 September 2024.
References

References



Prevalence

