

This infographic is supported by Accord Healthcare Ltd.
Cardiovascular Risk and
Urology


Introduction

PCa is the second-most prevalent cancer and the fifth-leading cause of cancer death amongst men.1
The incidence of PCa is increasing globally.2
ADT remains the mainstay approach to PCa management, achieved medically by treatment with GnRH agonists, GnRH antagonists, or androgen pathway inhibitors.3
GnRH agonists are the most frequently used type of ADT. However, these agents have been associated with an increased risk of CVD.3
Estimated number of global new cases of PCa from 2022 to 20456


CV effects of GnRH antagonists versus agonists in PCa
Although data are sparse, clinical trials and �meta-analyses suggest that GnRH antagonists may offer a more favourable cardiovascular toxicity (Cf EAU guidelines 7.2.3.4 Monitoring of metabolic complications) than GnRH agonists for patients at high risk of CV disease.3,5-8��
This difference might be due to the different modes of action of agonists and antagonists, although the exact mechanism is not currently clear.9��
The ESC 2022 Guidelines on Cardio-Oncology recommend GnRH antagonists for patients with pre-existing symptomatic CAD who require ADT.9��
Similar recommendations are included in the EAU 2024 guidelines on PCa, which suggest that GnRH antagonists should be considered in patients with pre-existing CVD or other CV risk factors if chemical castration is chosen.10��
Recent RWE studies did not replicate the results found in clinical trials.11However, the retrospective nature of these studies may have contributed to a selection bias whereby HCPs were more likely to prescribe a GnRH antagonist to patients with advanced disease or a CV history.11

seem to suggest that GnRH antagonists were associated with lower MACE risk and non-significantly decreased mortality risk compared with GnRH agonists.
Meta-analysis of 11 randomised trials (N=4,248)6 �
Systematic review of RCTs evaluating GnRH antagonists versus agonists6
MACE: RR 0.57 (95% Crl: 0.37–0.86) All-cause mortality: RR 0.58 (95% Crl: 0.32-1.06)
This study included RCTs evaluating (GnRH) antagonists and agonists among patients with PCa. Additional meta-analyses reported similar results. �

Reduced risk


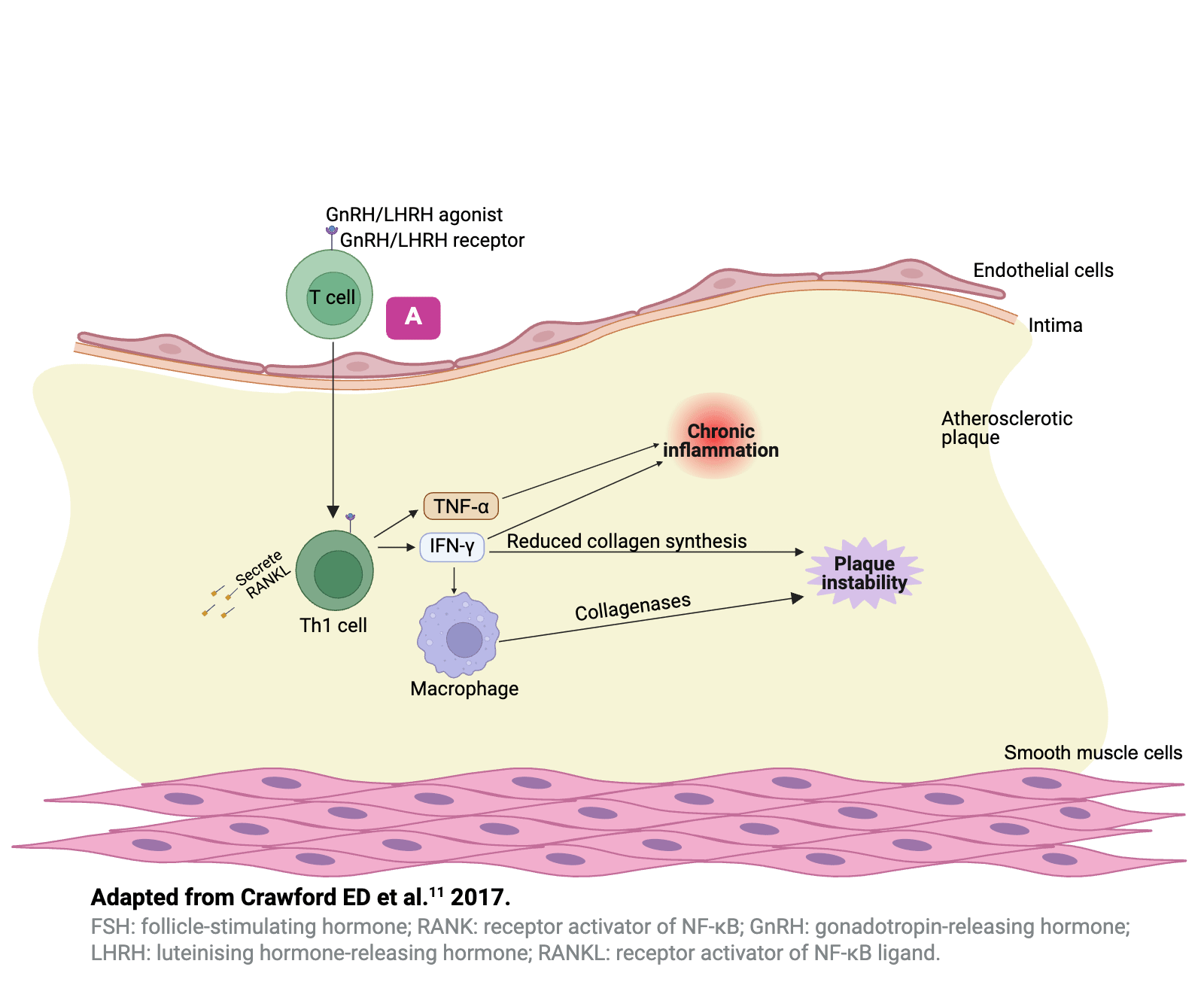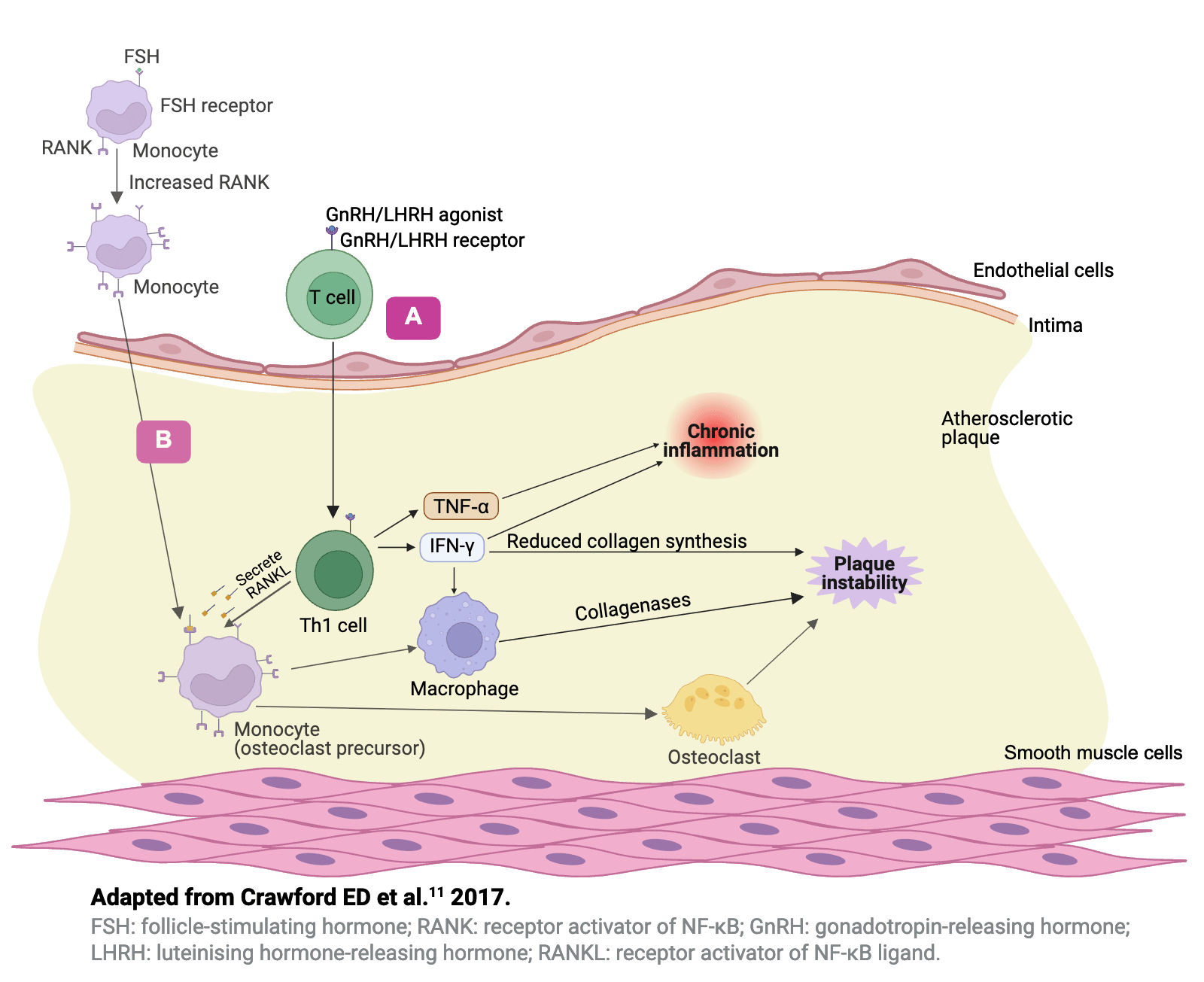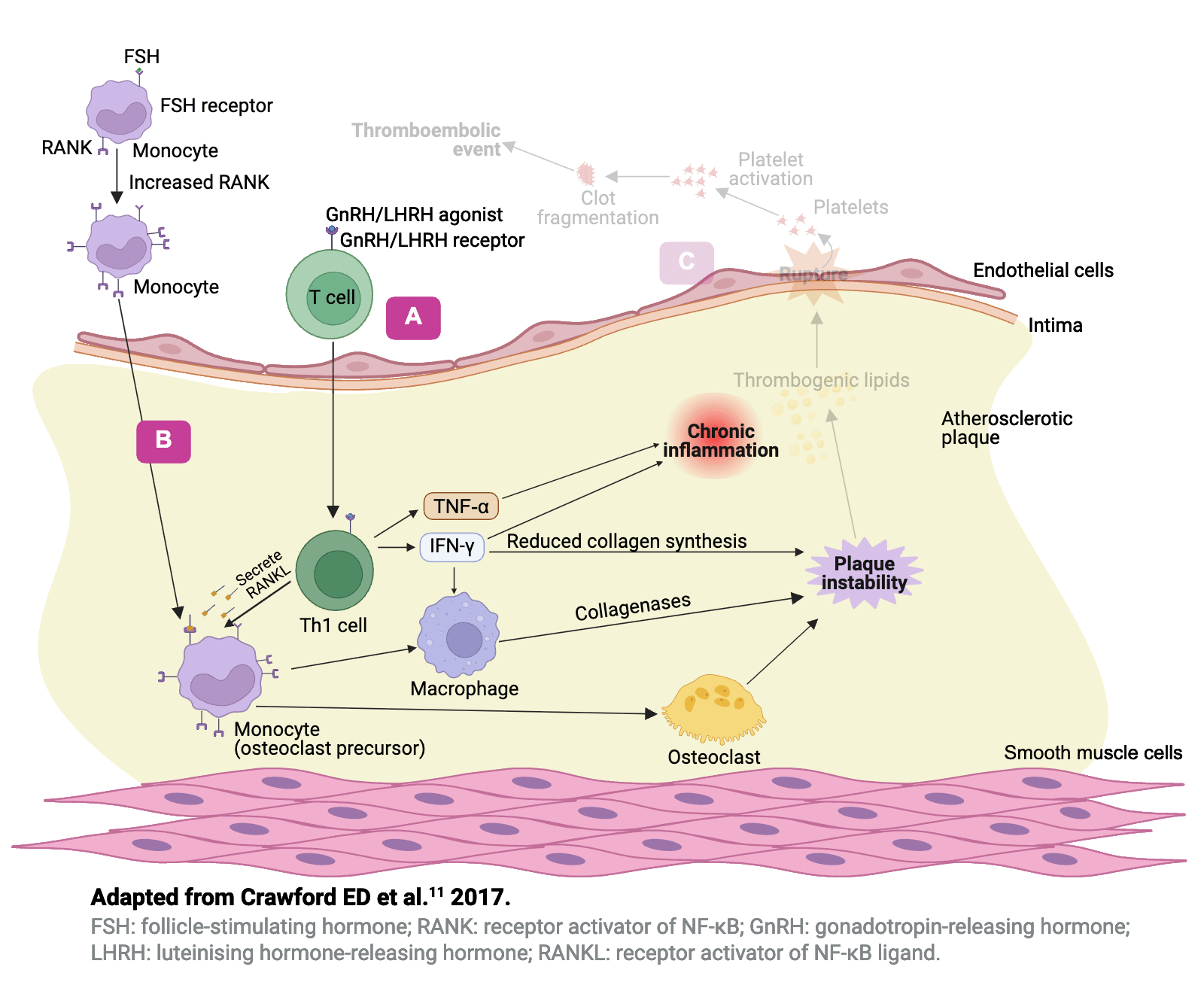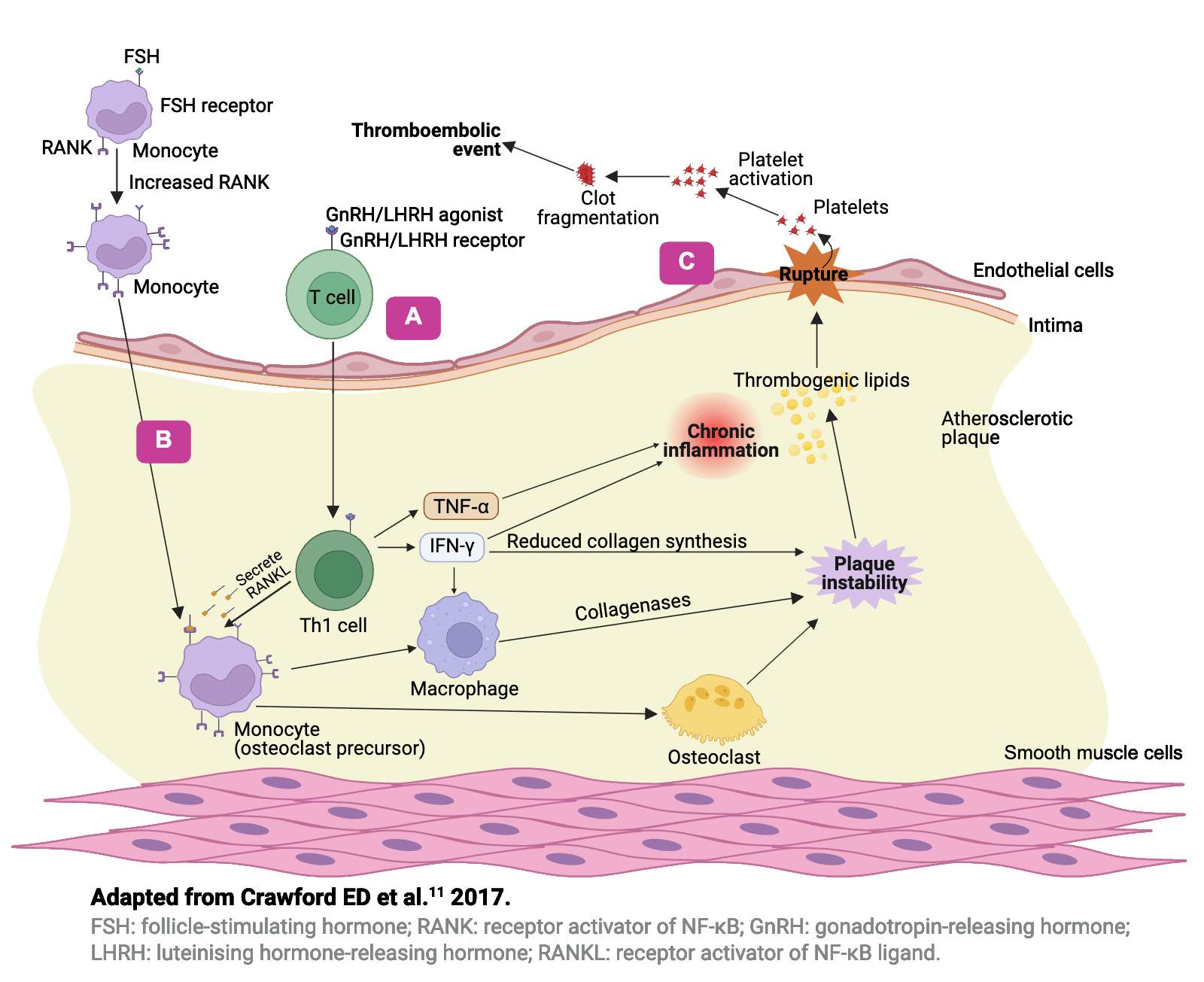
Hypotheses to explain the differential effects�of GnRH agonists and antagonists11
Checklist for CVD risk assessment and stratification
In 2023, the PCCV Expert Network panel developed a simplified and objective CV risk assessment tool to be used by urologists before initiating ADT treatment for PCa.3��
This tool provides a practical framework that allows urologists to promptly estimate a patient’s risk of CVD based on common CV risk factors.3��
Patients are stratified as having low, intermediate, or high risk of CVD.3





Minimising cumulative CVD risk at ADT initiation
ESC Guidelines recommend that physicians customise PCa treatment according to the CV risk factors of patients, taking into account the CV toxicities of individual ADTs.3,6
CVD risk category per checklist
High CVD risk
1. Refer to cardiologist for full cardiovascular assessment, and
2. Consult with cardiologist for PCa treatment plan, and
3. Initiate GnRH antagonist for ADT, and
4. Provide CVD risk education and emphasise the importance of risk management
Intermediate CVD risk
1. Consider a GnRH antagonist for ADT, and
2. Consider shorter duration of ADT, and
4. Refer to general practitioner/family physician to optimise CVD risk factors, and
3. Provide CVD risk education and emphasise the importance of risk management, and
5. If the patient has a CV event, refer to a cardiologist and consider medication review*
1. Monitor CVD risk, and
2. Consider a GnRH agonist or antagonist for ADT, and
3. Provide CVD risk education and emphasise the importance of risk management, and
4. lf patient develops any CVD risk factors, refer to general practitioner/family physician
Low CVD risk














Adapted from Merseburger et al. 20243�*Referral to cardiologists is recommended but is subject to each country’s healthcare system and resources.


Conclusion
There is increasing recognition of the need to adapt current PCa treatment strategies to account for comorbidities, particularly pre-existing CVD or CV risk factors.3
The long-term management of PCa should involve awareness of CV risk factors and the implementation of a routine CV risk assessment during consultations.3
It is recommended that patients with PCa and high CVD risk should be treated with a GnRH antagonist.3,9,10�Relugolix is part of the GnRH antagonist class.
Patients with high CVD risk may also require close, specialised CV monitoring and care devised by a cardiologist and communicated to the healthcare team.3
CV surveillance and vigilance should be maintained throughout the course of ADT treatment.3

1. Sung H et al. CA Cancer J Clin. 2021;71(3): 209-49.
2. Schafer EJ et al. Eur Urol. 2024:S0302-2838(24)02707-6.
3. Merseburger AS et al. World J Urol. 2024;42(1):156.
4. World Health Organisation, International Agency for Research on Cancer. 2024. Available at: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=27&single_unit=50000. Last accessed: 14 October 2024.
5. Boland J et al. Curr Cardiol Rep. 2021;23(8):109. 6. Nelson AJ et al. JACC CardioOncol. 2023;5(5):613-24.
7. Gu L et al. Front Endocrinol (Lausanne). 2023;14:1157857.
8. de Moraes FCA et al. Clin Transl Oncol. 2024 Nov 5. doi: 10.1007/s12094-024-03772-2. Epub ahead of print. PMID: 39500846.
9. Lyon AR et al. Eur Hear t J. 2022;43(41):4229-361.
10. EAU Guidelines. Edn. presented at the EAU Annual Congress Paris April 2024. ISBN 978-94-92671-23-3. Available at https://www.sciencedirect.com/science/article/pii/S0302283824027076: 28 October 2024. 11.Crawford ED et al. Urol Oncol. 2017;35(5):183-91.
References & Abbreviations:

ADT: androgen deprivation therapy; CAD: coronary artery disease; CrI: credible interval; CV, cardiovascular; CVD: cardiovascular disease; EAU: European Association of Urology; ESC: European Society of Cardiology; GnRH: gonadotropin-releasing hormone; MACE: major adverse cardiovascular event; NHA: novel hormonal agent; OR: odds ratio; PCa: prostate cancer; PCCV: Prostate Cancer Cardiovascular Expert Network; RCT: randomised clinical trial; RR: relative risk.
Abbreviations:
References & Abbreviations:
EUR-Onc-Org-01477 | June 2025









































Adapted from Merseburger et al. 20243 A minimum of one check-marked condition is needed to select “Yes” in a subsequent text box.�*A patient’s risk level may transition from “Low Risk” to “Intermediate Risk” or “High Risk” after 2 or 3 years of hormonal plus NHA treatment.

ADT in Prostate Cancer

If you are a HCP in the EU, prescribing information can be found here
If you are a HCP in the UK, prescribing information can be found here
Adverse events should be reported. For UK healthcare professionals, reporting forms, and information can be found at https://yellowcard.mhra.gov.uk/.Adverse events should also be reported to Accord-UK LTD on 01271 385257 or email medinfo@accord-healthcare.com.
For non-UK/EU healthcare professionals, you can report side effects directly via the national reporting system listed in Appendix V of the EU SmPC.
▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Forest plot of ORs and corresponding 95% (Cri) for (GnRH) antagonists and (MACE) compared with GRH agonists. *Data abstracted from www.clinicaltrials.gov. HERO: A Study to Evaluate the Safety and Efficacy of Relugolix▼ in Men With Advanced Prostate Cancer; PRONOUNCE: A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease. A total of 152 patients with primary outcome events were observed, 76 of 2,655 (2.9%) in GnRH antagonist-treated participants and 76 of 1,593 (4.8%) in agonist-treated individuals.