

Neurology

This interactive infographic has been funded by Novo Nordisk.
Alzheimer’s Diagnosis Dilemma:
How Do You Confirm
the Presence of Amyloid?
INTRODUCTION
How would you confirm amyloid pathology?
Otto (73 years old) is experiencing mild cognitive impairment (MCI).

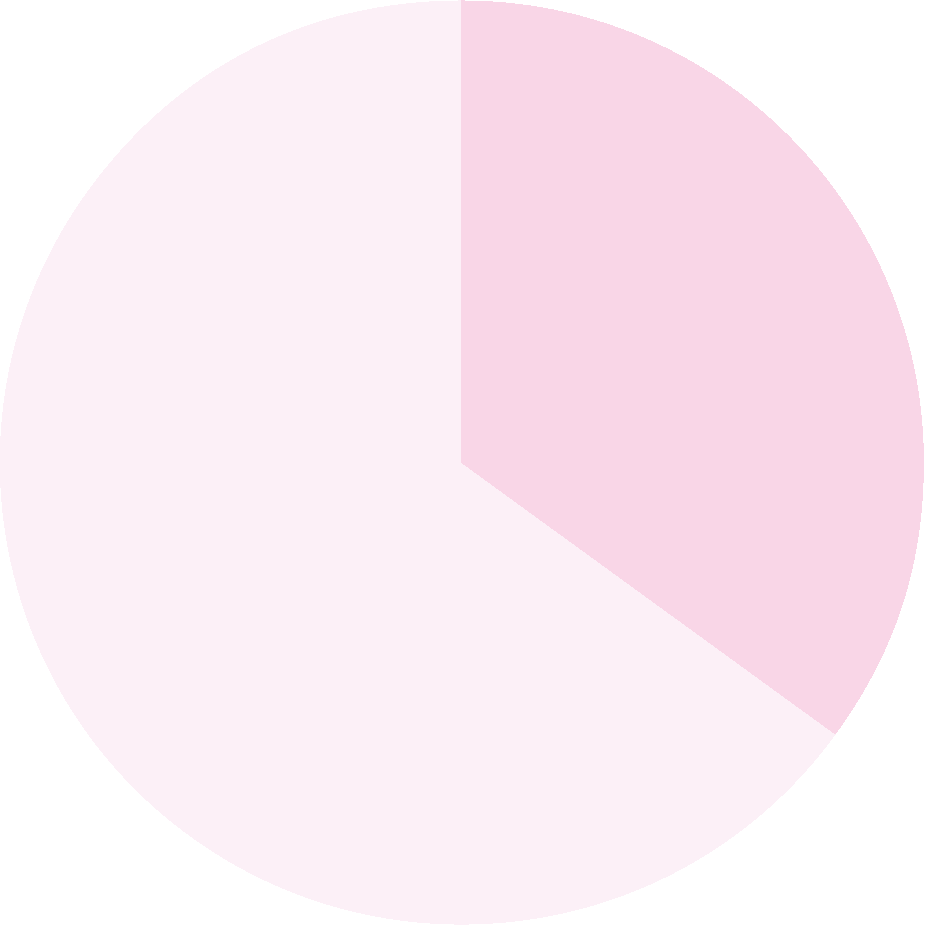

of patients are inaccurately diagnosed1
A clinical diagnosis based on cognitive symptoms alone can be unreliable in diagnosing AD.1
In a primary care setting, a clinical diagnosis of AD was inaccurate in 39% of patients.1
Delays in achieving a diagnosis can delay care and risk progression of the disease.2


How can we confirm amyloid pathology?
95%* of patients want simple testing if they are symptomatic,2 and confirmation is becoming increasingly necessary for treatment of AD or enrollment into clinical trials.1,3,4

CSF Biomarkers

Blood-based biomarkers (BBBMs)

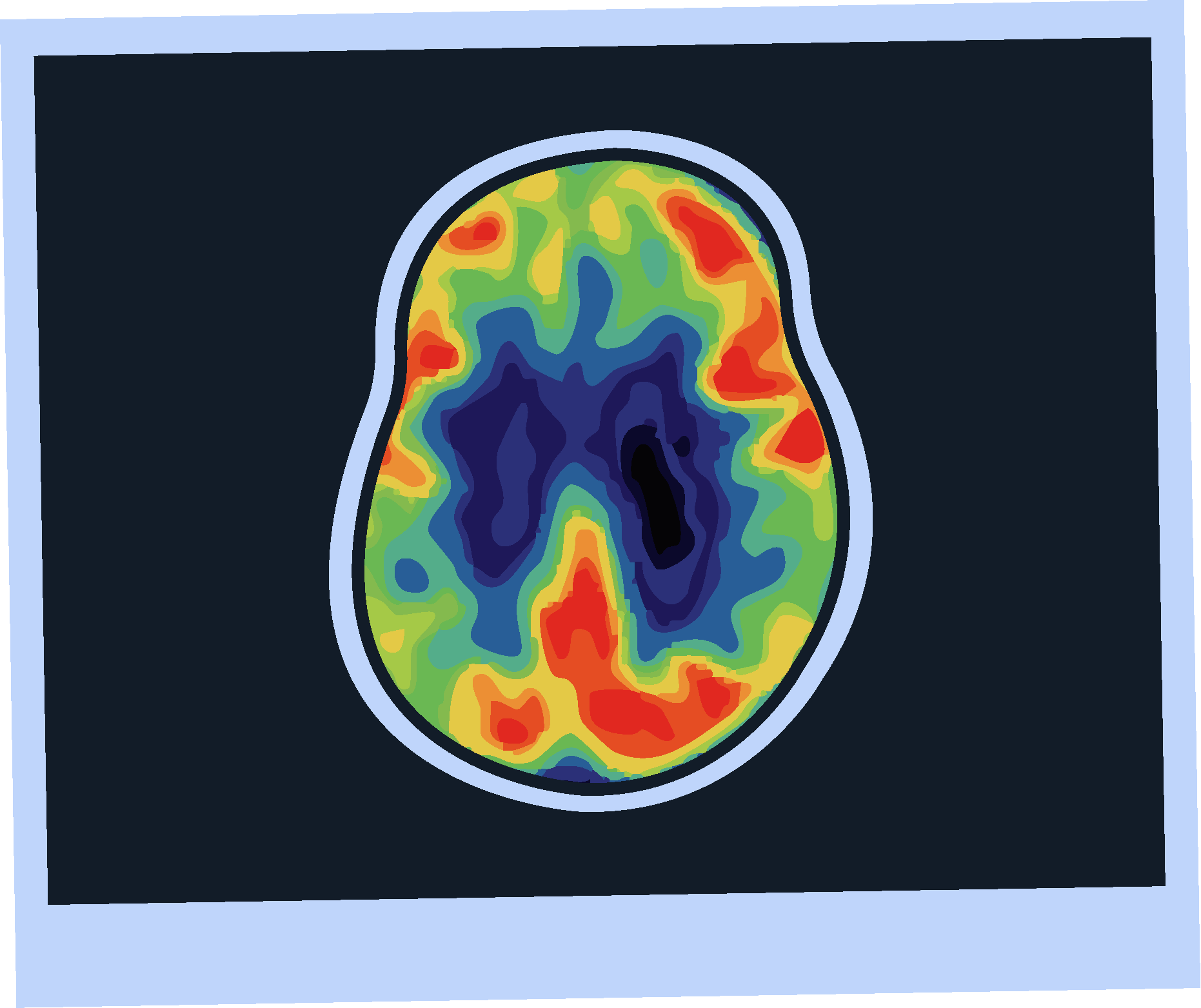
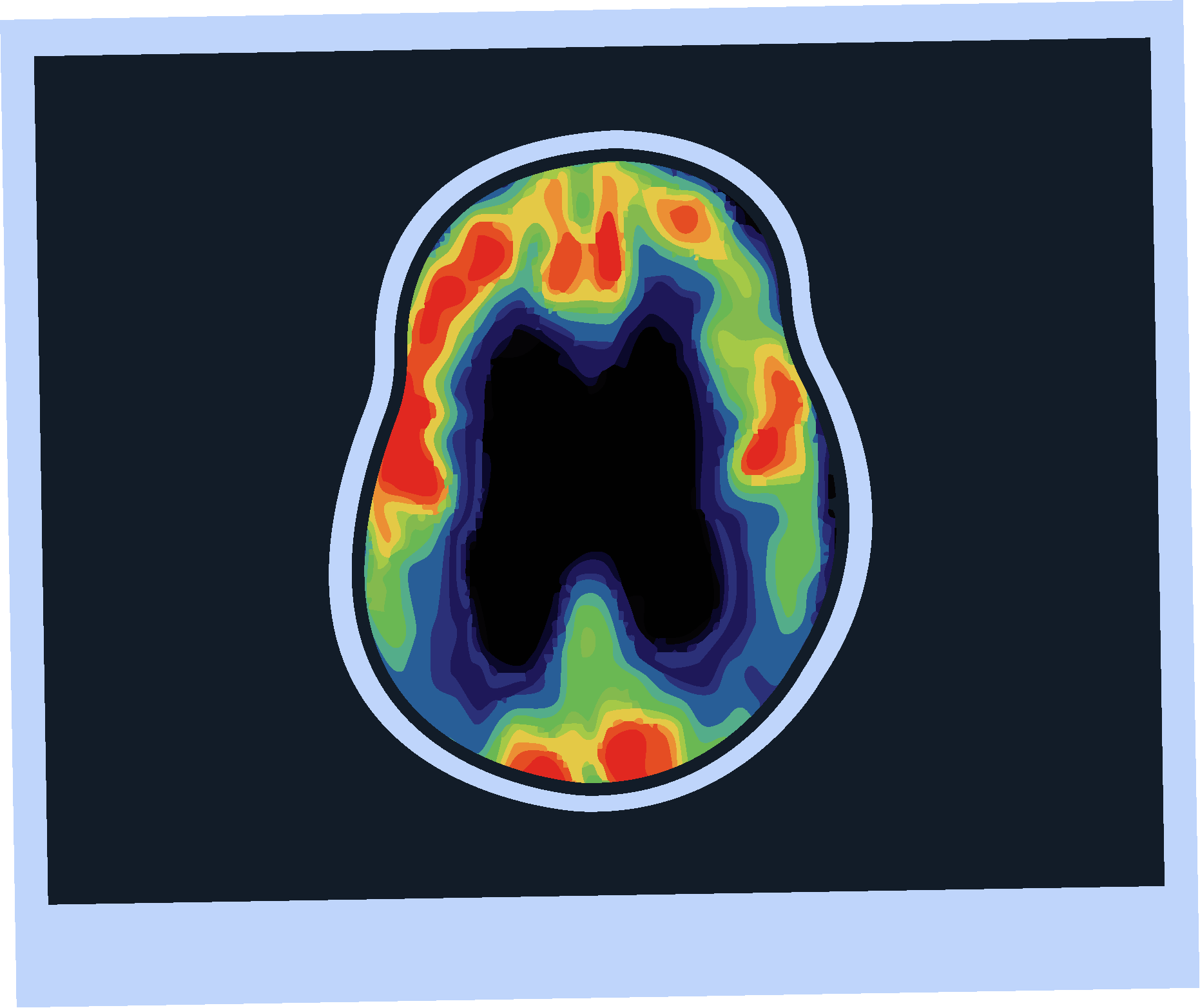
Amyloid PET Imaging
BBBMs
Blood testing allows amyloid status evaluation using plasma markers.1,6
Several studies have suggested that plasma ptau217 and the ptau217/Aβ42 ratio could have sufficient accuracy for use in symptomatic patients.7,8

Strengths
Limitations
Accuracy >90%; comparable to CSF ratios.1,7
Minimally-invasive, accessible, and scalable.7
ptau217/Aβ42 ratio has recently been FDA cleared for use in symptomatic patients.9
Endorsed by the 2024 Alzheimers�Association Workgroup.3
Approved for use only in patients with symptoms, not in the cognitively unimpaired.7
Limited real-world experience and�clinician familiarity.3,7
Potential for intermediate results.7
Intended for use by specialists.7

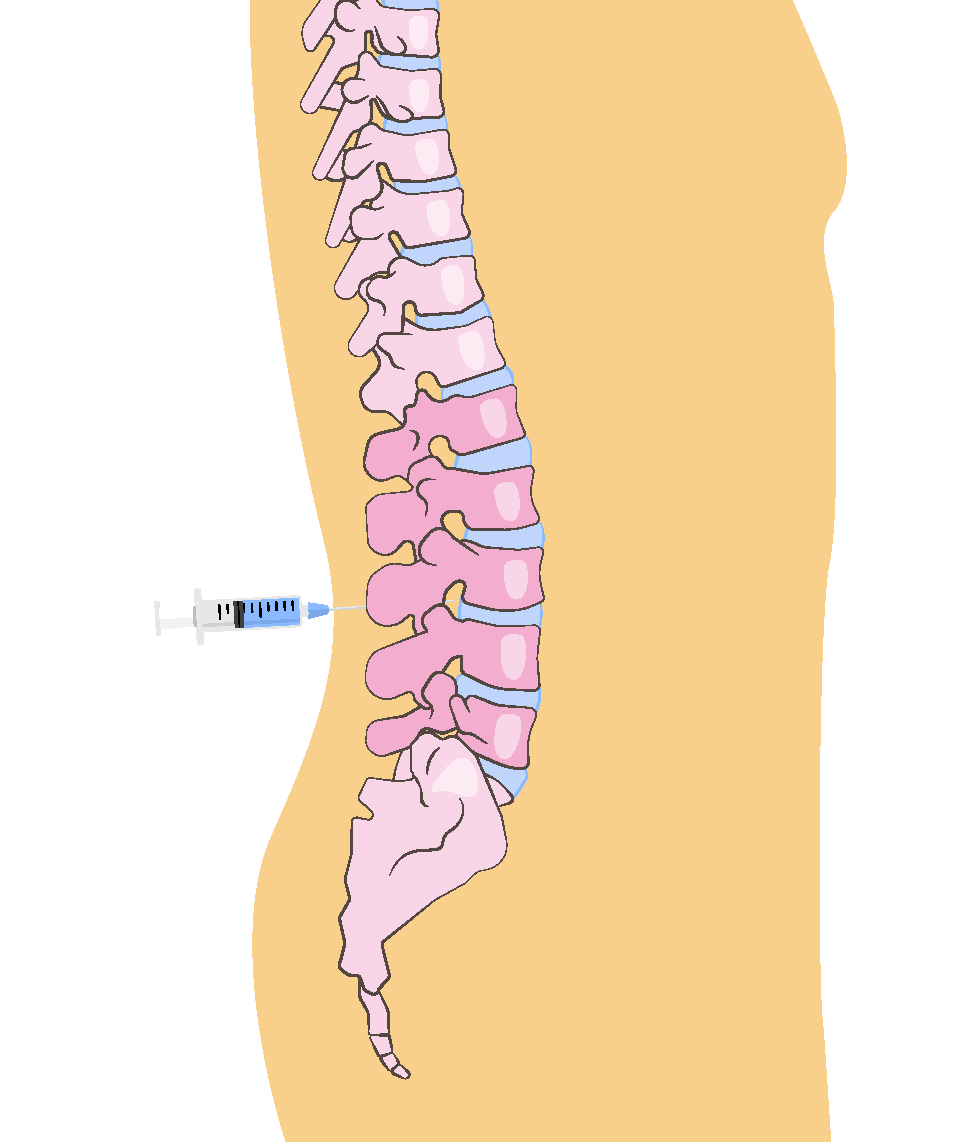
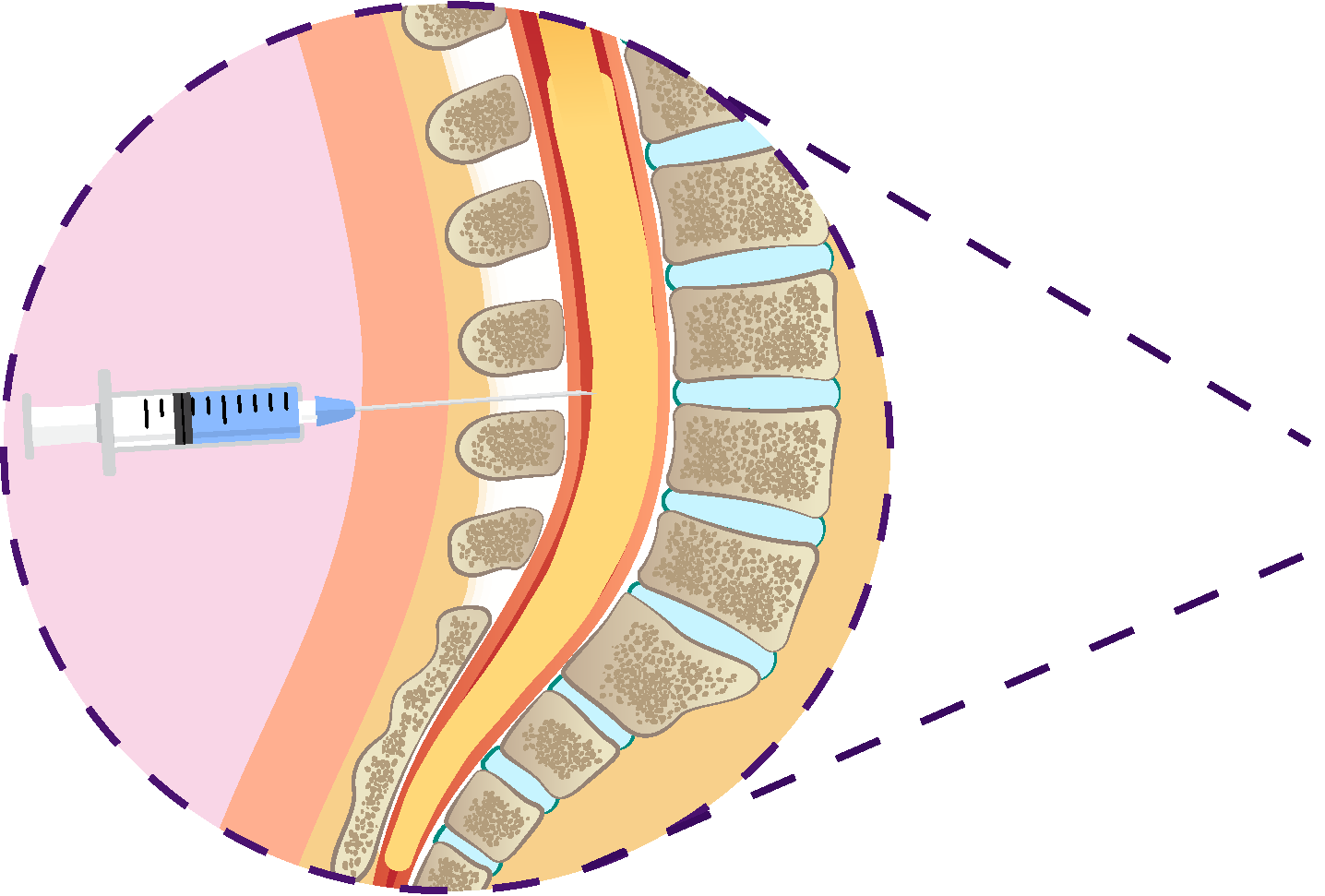
CSF Biomarkers

Strengths
Limitations
Hybrid ratios of analytes have sensitivity and specificity >90%.4
FDA approved and 2024 guideline endorsed.5
Measures multiple analytes that can expand the assessment of cognitive impairment.4
Broadly available and relatively low cost.4
Invasive procedure.4
Requires a highly trained clinician.4
Results can be influenced by comorbidities.4,5

Amyloid PET Imaging
Strengths
Limitations
Sensitivity and specificity >90% for AD pathology.4
FDA approved and 2024 guideline endorsed.5
Minimally invasive.4
Validated versus post-mortem pathology.4
Limited availability.4
Involves radiation exposure.4
Does not assess other causes of cognitive impairment.4
Subject to inter-rater variability.4


KEY TAKEAWAYS
The majority of patients with Alzheimer’s disease want a simple diagnostic test.2
Current and future disease modifying therapies for AD require confirmation of amyloid for initiation of therapy.1,5
Ultimately, the diagnostic approach should be selected based on the individual patient and clinical context.4


QUESTIONS
1. What is the diagnostic accuracy of a clinical diagnosis of AD in primary care?
A.
12%
C.
61%
B.
85%
D.
5%
2. Which of these are hallmarks of AD? (select all that apply)
A.
Beta amyloid protein
C.
Neuroinflammation
B.
Tau protein
D.
Brain atrophy
3. Which of these methods can be used to confirm the presence of amyloid in AD?
A.
BBBMs, urine sample, CT scan
C.
PET imaging, BBBMs, CSF biomarkers
B.
PET imaging, CSF biomarkers, X-ray
D.
CSF biomarkers, ultrasound, PET imaging
*A survey of 1,702 U.S. adults aged 45 years and older conducted from November 7–18, 2024, by the Alzheimer’s Association.2


Abbreviations


References
Hallmarks of Alzheimer’s disease include:2,4
accumulation of β-amyloid protein outside neurons
twisted strands of tau protein�inside neurons
inflammation and atrophy of brain tissue


As Otto is symptomatic, he may be able to undergo a minimally invasive blood test to assess his amyloid status. BBBMs have recently been cleared by the FDA for use in symptomatic patients, making this an emerging tool for use in clinical practice.

Otto suffers from both diabetes and hypertension, so his CSF biomarker results may potentially be influenced by these comorbidities.4,6


Otto lives in a rural area, where PET scans may not be widely available, he may have to travel a long distance to undergo amyloid PET imaging.
One study found that the mean one way travel time to a PET facility was 69 minutes.10

References
1. Palmqvist S et al. JAMA. 2024;332(15):1245-57.
2. Alzheimer’s Association. Alzheimers Dement. 2025;21:e70235.
3. Jack CR Jr et al. Alzheimers Dement. 2024;20(8):5143-69.
4. Leuzy A et al. Alzheimers Dement. 2025;21(3):e14528.
5. Atri A et al. Alzheimers Dement. 2024;DOI: 10.1002/alz.14333.
6. Bouteloup V et al. Neurology. 2024;102(8):e209219
7. Schöll M et al. J Prev Alzheimers Dis. 2025;12(4):100056.
8. Palmqvist S et al. Nat Med. 2025;31 (2036-2043).
9. Rubin R et al. JAMA. 2025;334(3):195-197
10. Li N, et al. Alzheimers Dement. 2025;21(4):e70100.

Abbreviations
Aβ: amyloid beta; AD: Alzheimer’s disease; BBBM: blood-based biomarker;�CSF: cerebrospinal fluid; MCI: mild cognitive impairment.

A.
12%
C.
61%
B.
85%
D.
5%
A.
Beta amyloid protein2,4
C.
Neuroinflammation2,4
B.
Tau protein2,4
D.
Brain atrophy2,4
A.
BBBMs, urine sample, CT scan
C.
PET imaging, BBBMs, CSF biomarkers
B.
PET imaging, CSF biomarkers, X-ray
D.
CSF biomarkers, ultrasound, PET imaging







CSF biomarkers, ultrasound, PET imaging
PET imaging, BBBMs, CSF biomarkers