This infographic was funded by GSK. This information is scientific & non promotional in nature

on Type 2 Inflammation
IL-5 in Focus: A New View
Respiratory

To what extent do you agree with the statements below?
Severe asthma is a heterogeneous disease with diverse underlying mechanisms contributing to how it manifests.1
As more evidence emerges regarding the molecular,2 cellular,3 clinical,4 and nonclinical4 factors impacting outcomes, there is an opportunity to refine our approach to patient management.5
Emerging research has revealed that IL-5 affects not just eosinophils,�but also multiple other immune and structural cells that contribute�to Type 2 inflammation.6

1. Global Initiative for Asthma. 2025. Available at: https://ginasthma.org/2025-gina-� strategy-report/. Last accessed: 27 August 2025.
2. Guo L et al. Ann Med. 2024;56(1):2258926.
3. Denlinger LC et al. J Allergy Clin Immunol Pract. 2020;8(2):474-82.
4. Tomisa G et al. J Asthma Allergy. 2019;12:297-307.
5. Agusti A et al. Eur Respir J. 2016;47(2):410-9.
6. Buchheit KM et al. Allergy. 2024;79(10):2662-79.
7. Persson EK et al. Science. 2019;364(6442):eaaw4295.
8. Howell I et al. J Exp Med. 2023;220(7):e20221212.
9. Pelaia C et al. Front Physiol. 2019;10:1514.
10. Travers J, Rothenburg ME. Eosinophils in mucosal immune responses. Mucosal � Immunol. 2015;8(3):464-75.
11. Ueki S et al. J Allergy Clin Immunol. 2016;137(1):258-67.
12. Holgate ST. Allergol Int. 2008;57(1):1-10.
13. Balzar S et al. J Allergy Clin Immunol. 2005;115:110-7.
14. Hough KP et al. Front Med (Lausanne). 2020;7:191.
15. Siddiqui S et al. J Allergy Clin Immunol. 2023;152(5):1121-30.e10.
16. Russell RJ et al. Eur Respir J. 2024;63:2301397.
17. Dunican EM et al. J Clin Invest. 2018;128:997-1009.
18. Barretto KT et al. Allergy. 2020;75:2127-30.
19. Bajbouj K et al. Allergy. 2023;78:882-5.
20. Plikus MV et al. Fibroblasts: Origins, definitions, and functions in health and � disease. Cell. 2021;184(15):3852-72.
21. Yamada T et al. J Allergy Clin Immunol. 1998;101(5):677-82.
References

References

PSE-US-4233


IL-5 is one of the key cytokines involved in Type 2 inflammation
Strongly disagree

Disagree

Unsure

Agree

Strongly Agree

Strongly disagree

Disagree

Unsure

Agree

Strongly Agree

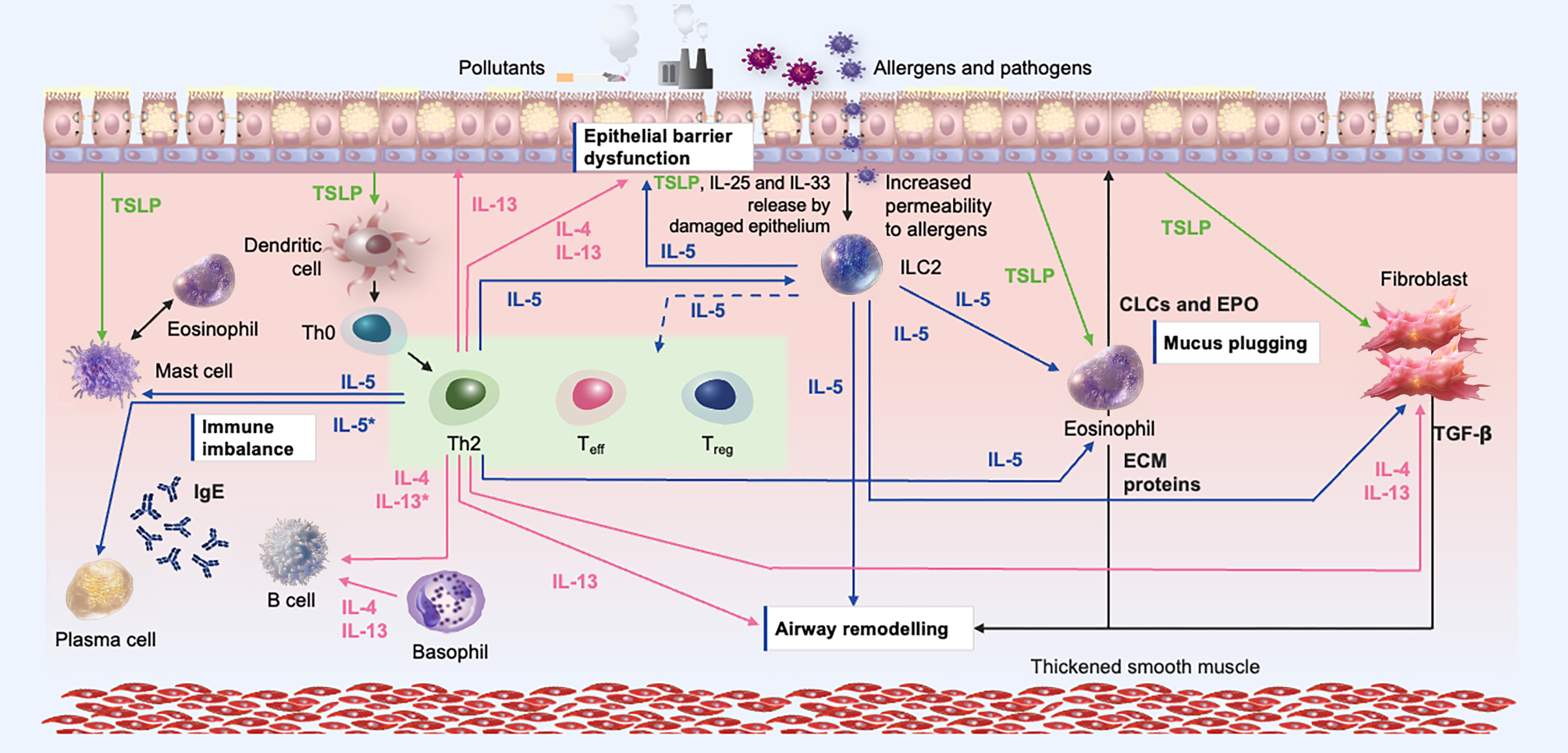
Mapping IL-5 Signaling and Its Clinical Consequences7,16-18,23-71



IL-5 is one of the key cytokines involved in Type 2 inflammation

IL-5 impacts other cells outside of eosinophils
IL-5 impacts other cells outside of eosinophils
Strongly Agree

Strongly Agree

Agree

Agree

Unsure

Unsure

Disagree

Disagree

Strongly disagree

Strongly disagree





IL-5 is a key cytokine involved in eosinophil inflammation as well as other cell processes
IL-5 is a key cytokine involved in eosinophil inflammation as well as other cell processes
Strongly Agree

Strongly Agree

Agree

Agree

Unsure

Unsure

Disagree

Disagree

Strongly disagree

Strongly disagree



Click to Meet the Cell Types Activated by IL-5 Signaling




Summary
INTRODUCTION
Emerging evidence suggests that IL-5 has additional effects beyond the modulation of eosinophilic inflammation, and multidirectional effects on cell types responsible for inflammatory processes.6
Understanding asthma’s molecular mechanisms will enable more precise treatments and better patient outcomes.
Watch this short video to learn more about Type 2 inflammation in severe asthma.

To what extent do you agree with the statements below?
Abbreviations & Resources



IL-5 is one of the key cytokines involved in Type 2 inflammation

IL-5 is one of the key cytokines involved in Type 2 inflammation
Strongly Agree

Strongly Agree

Agree

Agree

Unsure

Unsure

Disagree

Disagree

Strongly disagree

Strongly disagree



IL-5 impacts other cells outside of eosinophils

IL-5 impacts other cells outside of eosinophils
Strongly Agree

Strongly Agree

Agree

Agree

Unsure

Unsure

Disagree

Disagree

Strongly disagree

Strongly disagree



IL-5 is a key cytokine involved in eosinophil inflammation as well as other cell processes

IL-5 is a key cytokine involved in eosinophil inflammation as well as other cell processes
Strongly Agree

Strongly Agree

Agree

Agree

Unsure

Unsure

Disagree

Disagree

Strongly disagree

Strongly disagree



Mucus Plugging 1/2

Excessive mucus production can cause obstruction of the airways and reduce lung function.17
Eosinophilic activation by IL-5 can lead to mucus plug formation through EPO release, increased oxidation, and extracellular trap cell death.8,11,17







Eosinophilic activation by IL-5 also leads to IL-13 release73 and upregulation of MUC5AC in goblet cells by IL-13 results in the formation of mucus plugs in the lower airways.74
IL-5 inhibition reduces mucus plugging and airway obstruction in patients with severe asthma.75 Studies are ongoing to further understand how this relates to lung function and ventilation.76
Mucus Plugging 2/2



The MUC5AC gene is responsible�for mucin production and is more prevalent in cysteine domains, resulting in enhanced crosslinking and the formation of stiffer mucus gel.17




Airway Remodeling 1/2




IL-5 exposure also enhances proliferation and activation of fibroblasts from patients with asthma, suggesting that IL-5 may play a role in airway fibrosis and remodeling.19
Anti-IL-5 therapy reduced measures of airway remodeling in patients with severe asthma.28
Airway Remodeling 2/2


TGF-β is a potent regulator of fibroblast function, with the production of several extracellular matrix proteins.13,29,80


IL-5 contributes to airway remodeling through
multiple mechanisms.12,13,15,17-19,23,79
It is a significant driver of TGF-β release from eosinophils, which is central to airway remodeling.13,29,76,80

Healthy airway



Epithelial barrier dysfunction drives pathogenic processes, including impaired viral resistance and mucus hypersecretion, that have clinical implications for the patient.16,72
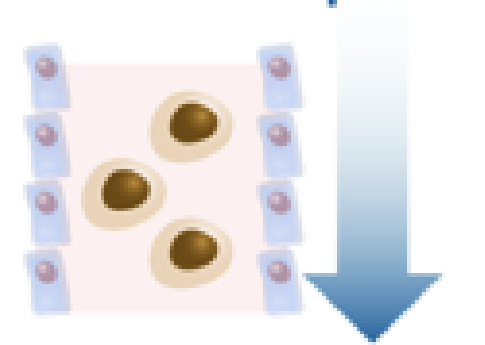
Epithelial barrier dysfunction 1/2



After Anti-IL5 Therapy

IL-5 suppresses the expression of tight junction genes, increasing permeability.18
This promotes mucociliary impairment and further inflammation.16 Inhibition of IL-5 significantly reduces bronchial epithelial damage in patients with severe asthma.28
Epithelial barrier dysfunction 2/2


Downregulation of genes relating to tight junctions and barrier function (eg, E-cadherin, caveolin) may increase epithelial susceptibility to damage and activation.18


Before Anti-IL5 Therapy




IL-5 plays a central role in Type 2 inflammation and is a key driver of both the adaptive and innate immune systems in severe asthma.9,77
In severe asthma, IL-5 is released from ILC2 cells and Th2 cells following activation by alarmins.9,77
Eosinophils
Immune imbalance 1/2




Dysregulated IL-5 orchestrates multidirectional effects on immune cells, resulting in immune imbalance.9,16,26,27,39,61,70,78
ILC2 activation status is downregulated following IL-5 inhibition in severe asthma.61
Epithelial barrier dysfunction 2/2


ILC2s from patients with severe asthma released more IL-5 and IL-13 compared with control groups, and IL-5 secretion by ILC2s was positively correlated with blood eosinophil levels.62






Meet the Cell Types Activated by IL-5 Signaling



















Ciliated epithelial
Eosinophils
Eosinophils: IL-5 directly affects eosinophils, contributing to mucus plugging, immune imbalance, airway epithelial barrier dysfunction, and airway remodeling in Type 2 inflammatory diseases.7-16




Ciliated epithelial cells: IL-5 can contribute to epithelial barrier dysfunction and mucus plugging in Type 2 inflammatory airway disease.6,16-18


Fibroblasts: IL-5 binds to and activates fibroblasts, which generate and deposit extracellular matrix proteins in the lamina propria, contributing to airway remodeling.6,19,20
Fibroblasts






Mast cells: IL-5 impacts immune imbalance through enhanced survival and/or proliferation of mast cell progenitors.6
Mast cells






Plasma: IL-5 directly influences plasma cell differentiation, survival, and Ig production, contributing to Type 2 immune imbalance and disease progression.6
Plasma






ILC2s: IL-5 indirectly affects ILC2, impacting immune imbalance and disease progression.6
ILC2s






Th2 and Treg cells: IL-5 indirectly affects T cells, contributing to immune imbalance and disease progression.6
Th2 & Treg cells






Neutrophils: IL-5 directly affects neutrophil activation, which impacts immune imbalance and disease progression in Type 2 inflammatory diseases.6
Neutrophils






B cells: IL-5 directly affects B cells, contributing to immune imbalance, Type 2 inflammation, and disease progression.6,8
B cells






BASOPHILS: IL-5 has demonstrated the ability to increase mediator release by human basophils, including augmenting the release of histamine due to other stimuli.21-23
BASOPHILS




Abbreviations & Resources
Abbreviations:
IL-5RA: interleukin 5 receptor alpha subunit; ILC2: Group 2 innate lymphoid cells; TGF-β: transforming growth factor-beta; Th2: Type 2 helper T cell; Ig: Immunoglobulin.
Extra Resources:
For the Type 2 Inflammatory Diseases Hub click here
Acknowledgements:
Medical writer assistance provided by Tom Gurney. Created by Tim Uden.


22. Hirai K et al. Enhancement of human basophil histamine release by� interleukin5. J Exp Med. 1990;172(5):1525-8.
23. Buchheit KM et al. J Allergy Clin Immunol. 2021;148:574-84.
24. Pelaia C et al. J Clin Med. 2023;12:3371.
25. Rakkar K et al. Am J Respir Crit Care Med. 2024;209:1268-72.
26. Bergantini L et al. Scand J Immunol. 2021;94:e13031.
27. Bergantini L et al. Biomed Pharmacother. 2023;166:115385.
28. Domvri K et al. Poster 3152. ERS Congress, 9-13 September, 2023.
29. Flood-Page P et al. J Clin Invest. 2003;112:1029-36.
30. Stirling RG et al. Am J Respir Crit Care Med. 2001;164:1403-9.
31. Shi H et al. Am J Respir Cell Mol Biol. 1997;16:220-4.
32. Takafuji S et al. Allergy. 1996;51:563-8.
33. Lee MH et al. Acta Haematol. 2013;130:238-41.
34. Rimkunas A et al. Eur Respir J. 2022;60(suppl 66):2842.
35. Luo J et al. Mucosal Immunol. 2024;17:524-36.
36. Koranteng J et al. Eur Respir J. 2023;62(suppl 67):PA552.
37. Xu J et al. Respir Med. 2007;101:1447-54.
38. Buchheit KM et al. J Allergy Clin Immunol. 2020;145:1574-84.
39. Sohail A et al. J Allergy Clin Immunol. 2024;153:527-32.
40. Wong CK et al. Am J Respir Cell Mol Biol. 2010;43:305-15.
41. Wu J et al. Cell Biochem Funct. 2013;31:496-503.
42. Buchheit KM et al. J Allergy Clin Immunol. 2016;137:1566-76.e5.
43. Kaur D et al. Chest. 2012;142:76-85.
44. Nagarkar DR et al. J Allergy Clin Immunol. 2013;132:593-600.e12.�45. Jiao J et al. Clin Exp Allergy. 2016;46:449-60.
References cont...



46. Laoukili J et al. J Clin Invest. 2001;108:1817-24.
47. Everman JL et al. Nat Commun. 2024;15:3900.
48. Thavagnanam S et al. Pediatr Res. 2011;69:95-100.
49. Nagashima H et al. Allergol Int. 2011;60:291-8.
50. Austin CD et al. Clin Exp Allergy. 2020;50:1342-51.
51. Bellini A et al. Mucosal Immunol. 2012;5(2):140-9.
52. Hashimoto S et al. J Allergy Clin Immunol. 2001;107(6):1001-8.
53. Batra V et al. Clin Exp Allergy. 2004;34:437-44.
54. Plante S et al. J Allergy Clin Immunol. 2006;117:1321-7.
55. Bergeron C et al. Clin Exp Allergy. 2003;33:1389-97.
56. Carsuzaa E et al. Int J Mol Sci. 2022;23:6308.
57. Mitamura Y et al. J Biol Chem. 2018;293:14646-58.
58. Ingram JL et al. Am J Respir Crit Care Med. 2011;183:1625-32.
59. Firszt R et al. Eur Respir J. 2014;43:464-73.
60. Ingram JL et al. Am J Respir Cell Mol Biol. 2016;54:41-50.
61. Malik B et al. Respirology. 2023;28:758-66.
62. Fieux M et al. Int Forum Allergy Rhinol. 2024;11(8):1137-49.
63. Wise SK. Int Forum Allergy Rhinol. 2014;4:361-70.
64. Richter A et al. Am J Respir Cell Mol Biol. 2001;25:385-91.
65. Soyka MB et al. J Allergy Clin Immunol. 2012;130:1087-96.e10.
66. Cho H-J et al. Allergy. 2023;78:1866-77.
67. Chen J et al. Int Immunopharmacol. 2023;121:110554.
68. Ji X et al. Int J Clin Exp Pathol. 2013;6:1481-92.
69. Wu C et al. Blood Adv. 2022;6:4439-49.
References cont...



70. Galdiero MR et al. Front Med (Lausanne). 2017;4:103.
71. Taillé C et al. Am J Respir Crit Care Med. 2024;209:A1051.
72. Raby KL et al. Front Immunol. 2023;14:1201658.�73. Uchida AM et al. Front Immunol. 2022;13:946643.�74. Dunican EM et al. Ann Am Thorac Soc. 2018;15(suppl 3):S184-S191.�75. Hamakawa M, Ishida T. Intern Med. 2024;63(22):3133-4.�76. Hannover Medical School. NCT04512521. https://clinicaltrials.gov/study/NCT04512521.�77. Maspero J et al. ERJ Open Res. 2022;8:00576-2021.�78. Guillet C et al. J Allergy Clin Immunol Pract. 2021;9:3846-47.�79. Frigas E, Gleich GJ. J Allergy Clin Immunol. 1986;77:527-37.
80. Minshall EM et al. Am J Respir Cell Mol Biol. 1997;17:326-33.
References