
Avelumab First-Line Maintenance Treatment for Advanced Urothelial Carcinoma: �Insights From Real-World Evidence in More Than 5,000 Patients


Funded by Merck
Acknowledgements: Medical writing support was provided by Sophie Saunders of Nucleus Global and was funded by Merck KGaA, Darmstadt, Germany.
Keywords: Avelumab, urothelial carcinoma, first-line maintenance, platinum-based chemotherapy, real-world evidence

Summary
Urothelial carcinoma (UC), a prevalent cancer affecting the urinary tract, often progresses to an advanced, terminal stage. Platinum-based chemotherapy has been a mainstay first-line (1L) treatment for patients with advanced UC for >20 years. Avelumab is a guideline recommended 1L maintenance treatment in patients with advanced UC without disease progression following 1L platinum-based chemotherapy. The pivotal JAVELIN Bladder 100 Phase III trial demonstrated that avelumab significantly improves overall survival (OS) and progression-free survival (PFS).
Real-world evidence from over 5,000 patients across more than 15 countries has corroborated these findings, showing consistent effectiveness and manageable safety in diverse populations. This includes patients who would have been ineligible for enrolment in JAVELIN Bladder 100, such as patients with an Eastern Cooperative Oncology Group (ECOG) performance status of ≥2, patients with various comorbidities, and patients who had received an alternative cisplatin-based chemotherapy regimen (such as dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin [ddMVAC]). Median OS in real-world studies ranged from 16.8 to 39.5 months. These studies support avelumab's role as a recommended 1L maintenance option and provide insights on treatment sequencing, with median OS of up to 3 years observed in patients who received a sequence of guideline-recommended 1L platinum-based chemotherapy followed by avelumab 1L maintenance in patients without disease progression and 2L enfortumab vedotin.




Summary



INTRODUCTION
TO UROTHELIAL CARCINOMA
Urothelial carcinoma (UC) is a cancer that arises from urothelial cells lining the bladder and urinary tract.1
According to the 2025 US �SEER database, approximately 13% of patients with UC already have advanced disease (i.e., locally advanced or metastatic) at diagnosis,4 which is considered terminal.
UC can also develop in the ureter, renal pelvis, and urethra.2
More than 90% of bladder cancers are UCs.1
Approximately 50% of patients with tumours that have spread into the muscle layer of the bladder wall ultimately develop metastatic disease.5
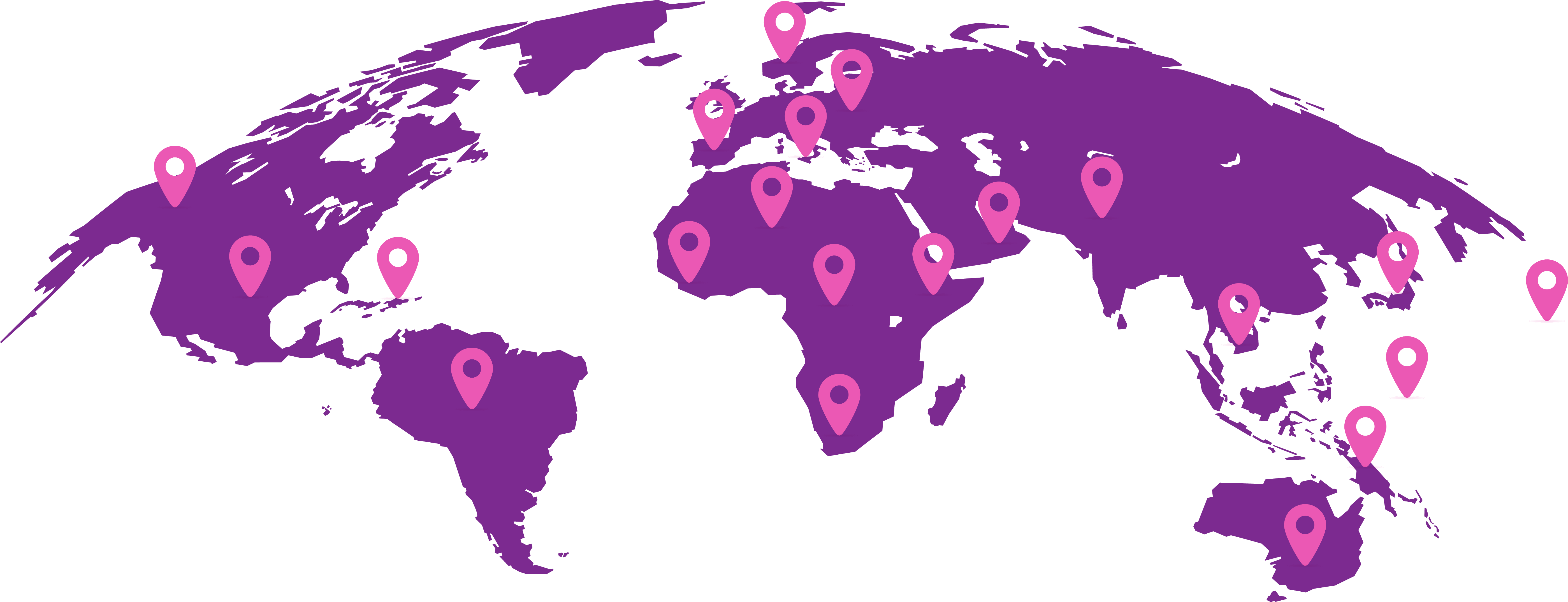
UC is a prevalent cancer, as shown by the fact that bladder cancer is the ninth most common cancer worldwide with an estimated >610,000 new cases diagnosed and >220,000 deaths in 2022.3

INCIDENCE RATES
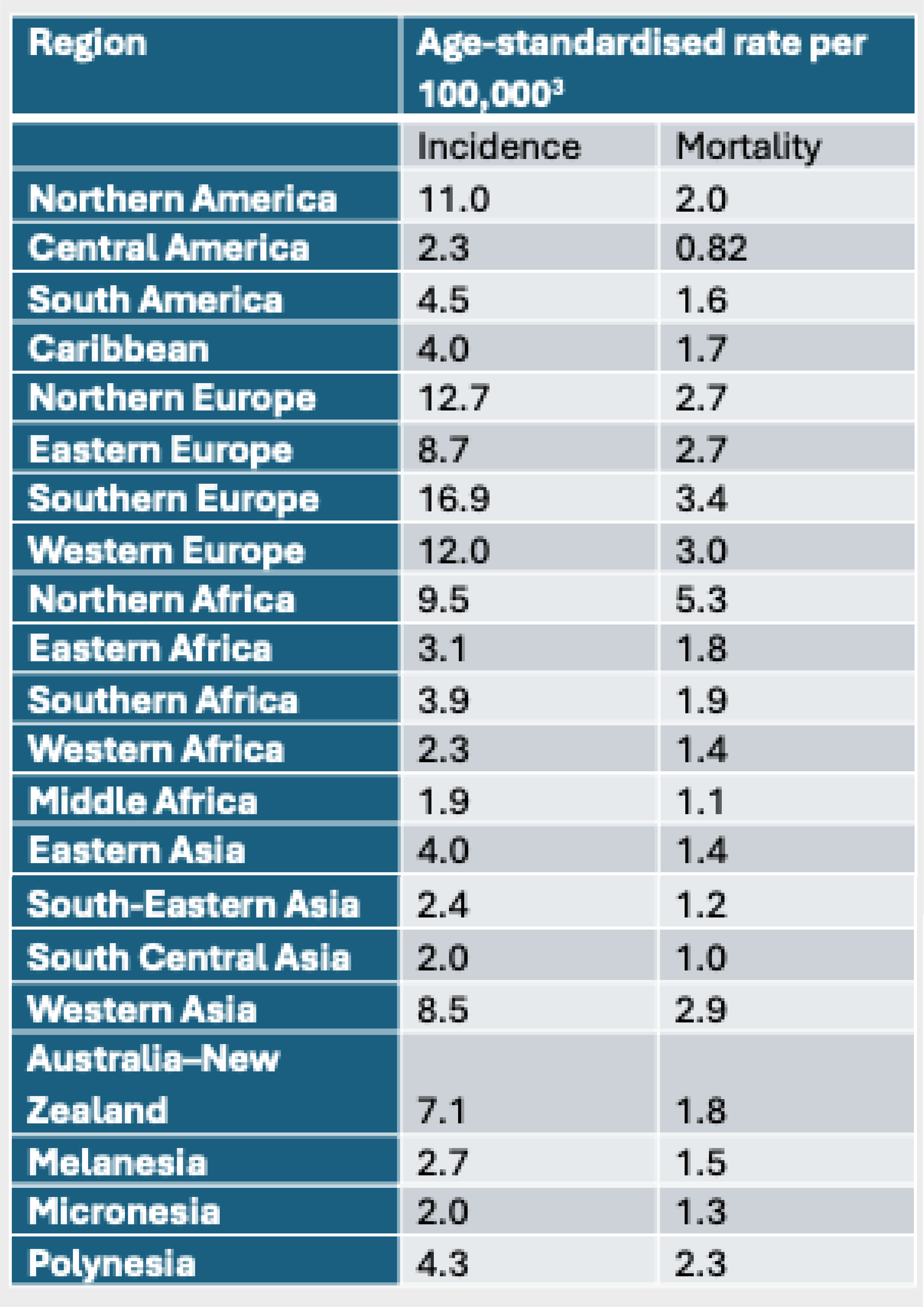
The highest incidence rates of bladder cancer were found in Southern, Northern, and Western Europe and North America, and over the next 20 years rates are predicted to increase worldwide.3
World map showing bladder cancer incidence rates in regions based on age-standardised rate per 100,0003
Incidence Rates
Undertreatment Rates
REAL-WORLD EVIDENCE FOR
AVELUMAB 1L MAINTENANCE TREATMENT
Real-world studies are important for confirming and extending the findings of clinical trials in heterogeneous patient populations seen in routine practice. To date, real-world studies of avelumab 1L maintenance have included >5,000 patients treated in >15 countries.25-55

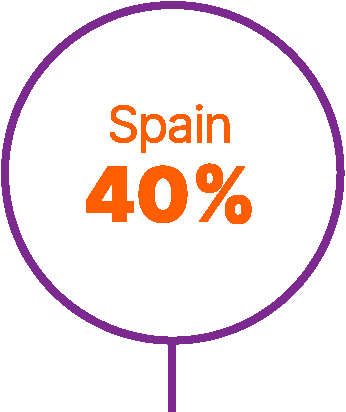
Total number of patients in real-world studies in different countries.25-55
REAL-WORLD STUDY OUTCOMES
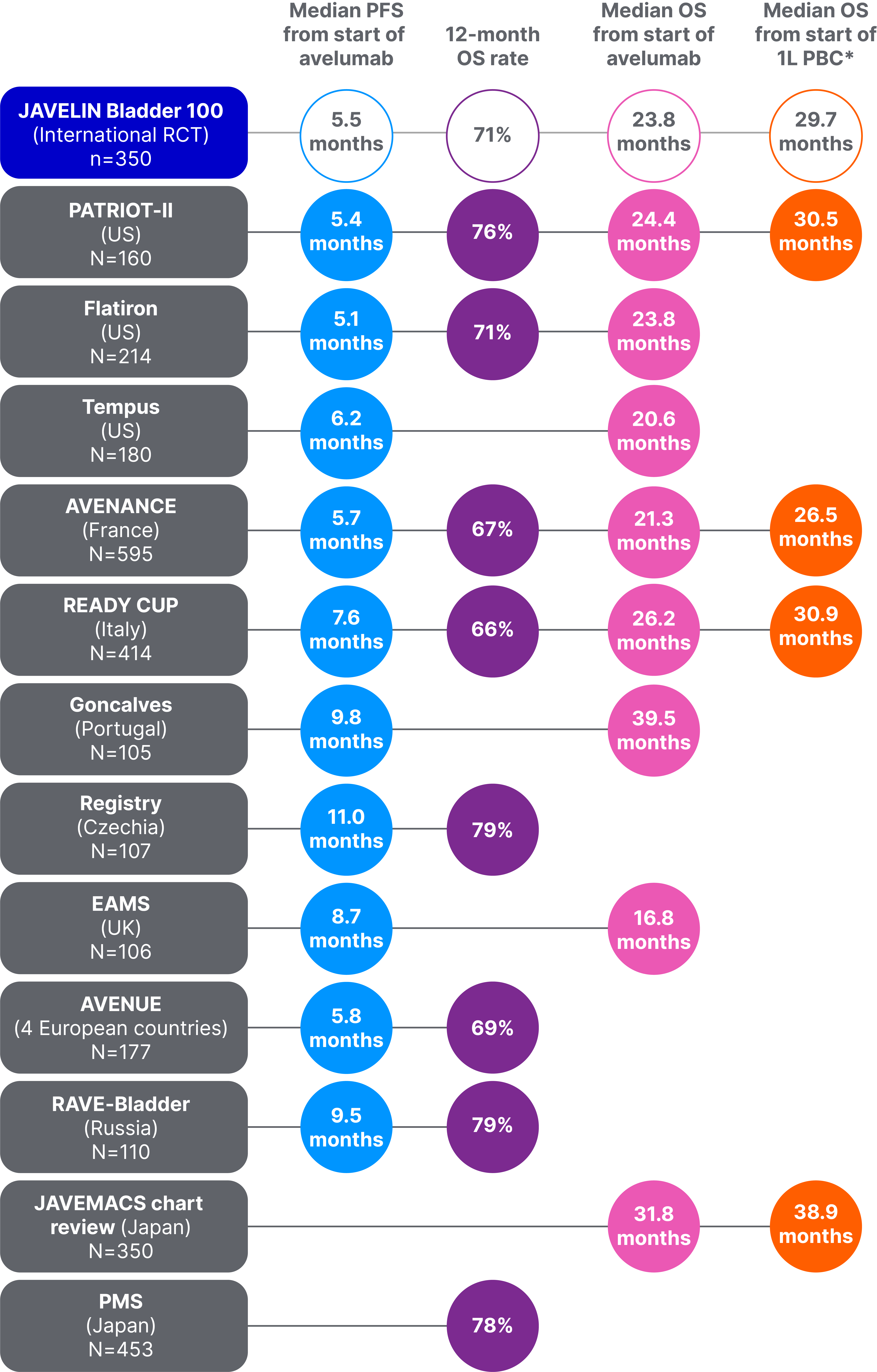
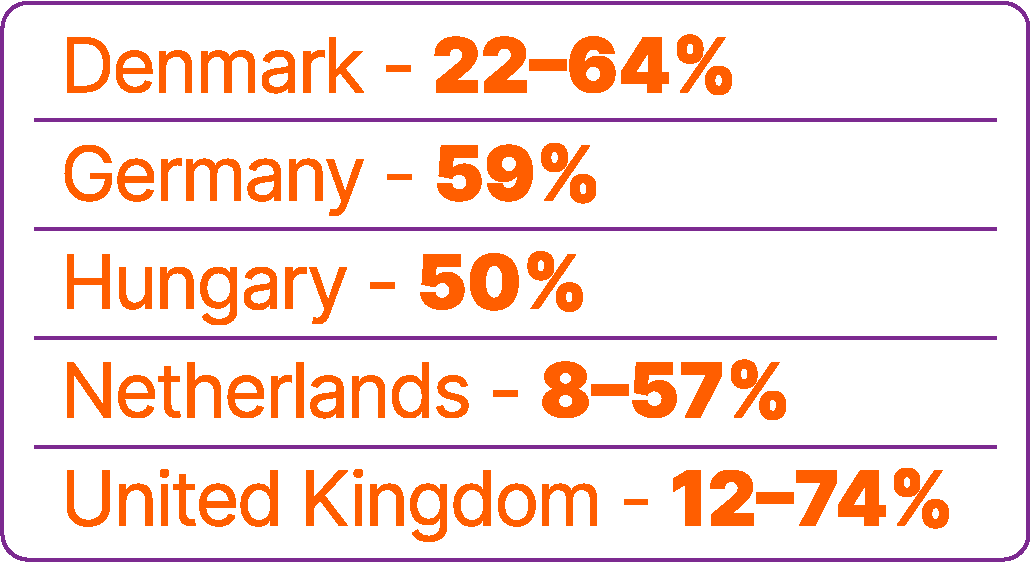
Overall, effectiveness and safety outcomes have been generally consistent across real-world studies and comparable to data reported from the JAVELIN Bladder 100 trial. Where reported in real-world studies, median OS from the start of avelumab ranged from 16.8–39.5 months,25-27,31,40,42,43,45,46,48 and median PFS ranged from 5.1–11.0 months.25-27,29,35,40,43,45-51,55
Median OS from the start of 1L platinum-based chemotherapy in these populations without disease progression after 1L platinum-based chemotherapy ranged from 26.5–40.6 months (limitations of this analysis are discussed below*).25,31,33,38,40,43
LIMITATIONS

INSIGHTS FROM LARGE REAL-WORLD STUDIES
Several real-world studies with large patient populations (>100 patients) have provided additional insights on avelumab 1L maintenance treatment.

AVENANCE40

READY CUP43

JAVEMACS31
Summary of survival data in patients treated with avelumab 1L maintenance from JAVELIN Bladder 100 and real-world studies (N>100): median progression-free survival, median overall survival, and 12-month overall survival rates from the start of avelumab first-line maintenance and median overall survival from the start of first-line chemotherapy.*17,18,25-27,31,32,40,43,45-47,49,51
Results across studies should not be compared due to differences in study design and patient populations.

CONCLUSION

Among treatments that have been shown to prolong survival in patients with advanced UC, extensive real-world evidence has confirmed the effectiveness and safety of avelumab 1L maintenance treatment in patients without progression after receiving 1L platinum-based chemotherapy.

Undertreatment Rates
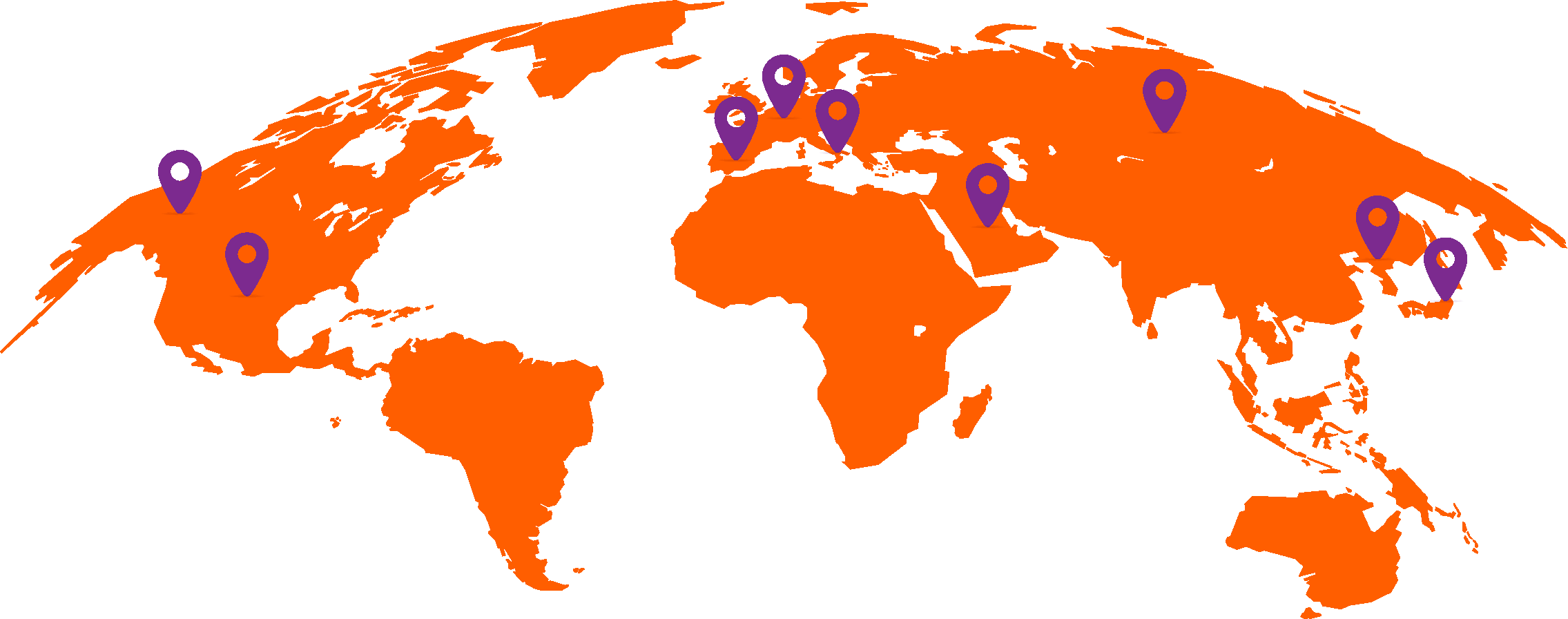
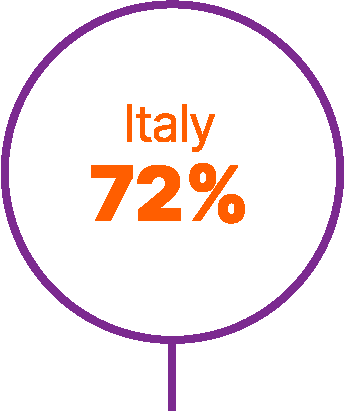
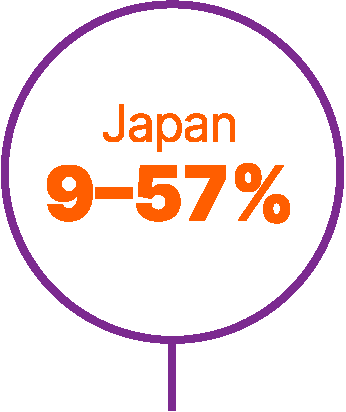
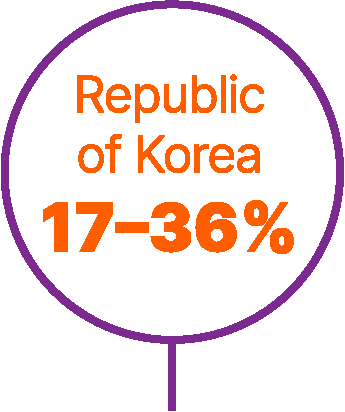
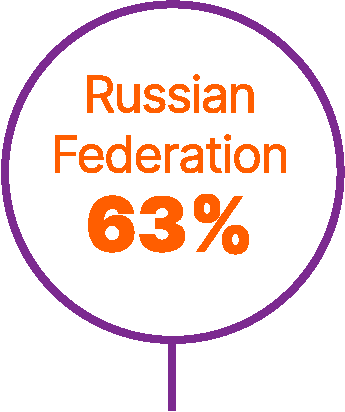
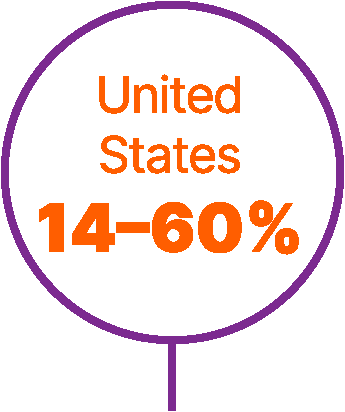
Approximately 40% of patients with advanced UC do not receive any systemic anticancer treatment.6
World map showing proportions of patients not receiving any systemic treatment for advanced urothelial carcinoma in different countries.6,9,56,57
Incidence Rates
Undertreatment Rates



In a retrospective study of a US electronic health record database (Flatiron Health), which included >8,000 patients with advanced UC between 2011 and 2020, untreated patients had short overall survival (OS; median 7 months).7


Studies have found that patients are less likely to receive systemic treatment if they are older, female, or have worse performance status, greater comorbidity, poor renal function, or visceral metastases.6,8,9
AVELUMAB 1L MAINTENANCE TREATMENT FOR ADVANCED UC


Platinum-based chemotherapy has been a mainstay first-line (1L) treatment for patients with advanced UC for >20 years.
Avelumab is an anti–programmed death ligand 1 immune checkpoint inhibitor that is recommended for 1L maintenance treatment in patients with advanced UC without progression following 1L platinum-based chemotherapy.2,15,16 Avelumab was first approved for this indication in 2020 based on results from the JAVELIN Bladder 100 Phase III trial.17,18


Study Design


Trial Results: Efficacy


Post Hoc Analysis

JAVELIN Bladder regimen

1L INDUCTION

1L MAINTENANCE
All endpoints were measured post randomisation (after chemotherapy)
Stratification �• Best response to 1L induction chemotherapy (CR or PR vs SD) �• Metastatic site (visceral vs nonvisceral [including bone metastasis])
Unresectable locally advanced or metastatic �UC with measurable stage IV disease
Received standard 1L chemotherapy (4-6 cycles)�• Cisplatin + gemcitabine or�• Carboplatin + gemcitabine
Treatment-free interval �4-10 weeks
(radiological assessment of response and AE resolution)
1:1
Patients with CR, PR, or SD
All patients: N=700
�PD-L1+ tumours: �n=358 (51%)
Avelumab 10mg/kg IV Q2W+BSC n=350
BSC alone n=350


Until PD, unacceptable toxicity, or withdrawal
Primary endpoint �• OS �- All randomised patients �- PD-L1+ population
Secondary endpoints �• PFS and objective response per RECIST 1.1 by BICR and investigator �• Safety and tolerability �• PROs

The trial enrolled 700 patients with unresectable locally advanced or metastatic UC and no disease progression (i.e., disease control) following 4–6 cycles of 1L platinum-based chemotherapy with either cisplatin plus gemcitabine or carboplatin plus gemcitabine.18

Enrolment

Following an interval of 4–10 weeks after the end of chemotherapy (to allow response assessment and resolution of any adverse events [AE]), patients were randomised 1:1 to receive avelumab plus best supportive care (BSC) or BSC alone.18


The primary endpoint of the study was OS measured from randomisation (i.e., the start of study treatment post chemotherapy).18


Abbreviations �1L: first line; AE: adverse event; BICR: blinded independent central review; BSC: best supportive care; CR: complete response; IV: intravenous; OS: overall survival; PD: progressive disease; PD-L1: programmed death ligand 1; PFS, progression-free survival; PR: partial response; PRO: patient-reported outcome; Q2W: every 2 weeks; RECIST: Response Evaluation Criteria in Solid Tumours; SD: stable disease; UC: urothelial carcinoma.

JAVELIN BLADDER 100 PHASE III TRIAL:

Results from JAVELIN Bladder 100 showed that avelumab plus BSC significantly prolonged OS and progression-free survival (PFS) compared with BSC alone.
After ≥2 years of follow-up in all patients, median OS from the start of study treatment was 23.8 vs 15.0 months (hazard ratio: 0.76 [95% CI: 0.63–0.91]; p=0.0036) and median PFS was 5.5 vs 2.1 months (hazard ratio: 0.54 [95% CI: 0.46–0.64]; p<0.0001), respectively.17

Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival measured from randomisation in the JAVELIN Bladder 100 study.17
Data cutoff: 4 June 2021. Median follow-up: 38.0 months in the avelumab plus BSC arm and 39.6 months in the BSC alone arm.17




Treatment benefits with avelumab 1L maintenance were generally consistent across prespecified and exploratory subgroups, including those defined by:
age,
level of renal function,
body mass index,
1L chemotherapy regimen, and
response to 1L chemotherapy (complete response, partial response, or stable disease).19-23
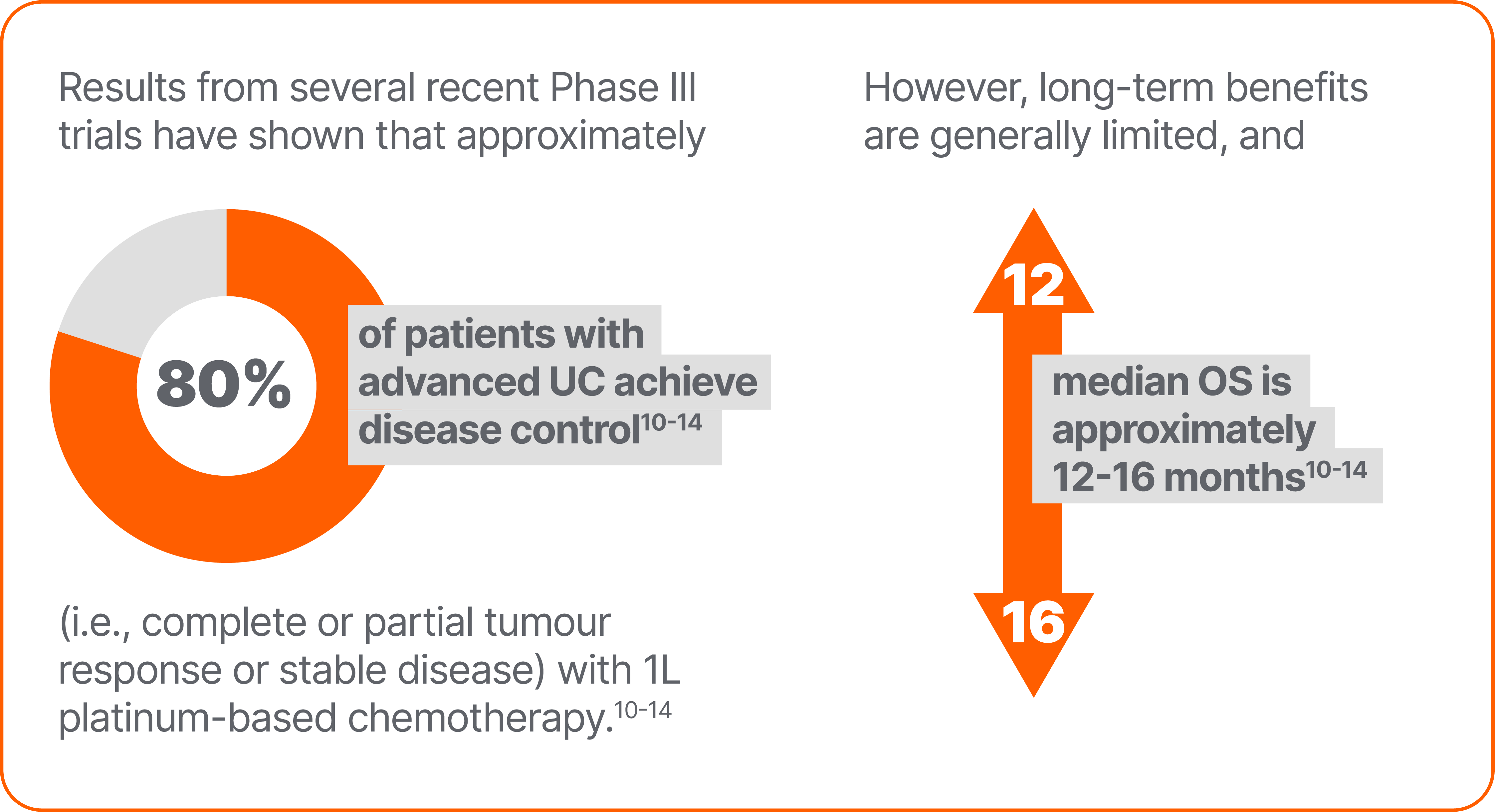
In an exploratory analysis in this trial population of patients without disease progression after 1L chemotherapy, median OS from the start of 1L chemotherapy was 29.7 months with avelumab plus BSC vs 20.5 months with BSC alone;19 this analysis should be interpreted with caution because of data limitations discussed below.*





Safety
Limitations


*Limitations of analyses of OS calculated from the start of 1L platinum-based chemotherapy in patients who received avelumab 1L maintenance include the following: analyses were performed in selected populations who remained progression free after 1L platinum-based chemotherapy; OS includes time receiving platinum-based chemotherapy (typically 4–6 cycles) and any interval between platinum-based chemotherapy and avelumab (typically 4–10 weeks); and OS includes immortal time bias because all patients analysed were required to have survived throughout 1L PBC to be eligible for avelumab 1L maintenance treatment. Thus, results should be interpreted with caution.

In safety analyses, the most common treatment-related AEs (TRAEs) were
pruritus (14.8%),
hypothyroidism (11.0%),
fatigue (10.8%),
asthenia (10.5%), and
diarrhoea (10.5%).17
TRAEs occurred in 78.2% of patients overall, including Grade ≥3 events in 19.5%, and 11.6% of patients discontinued avelumab treatment because of TRAEs.17
No new safety signals were identified with long-term treatment, including in patients treated for ≥12 months.17,18

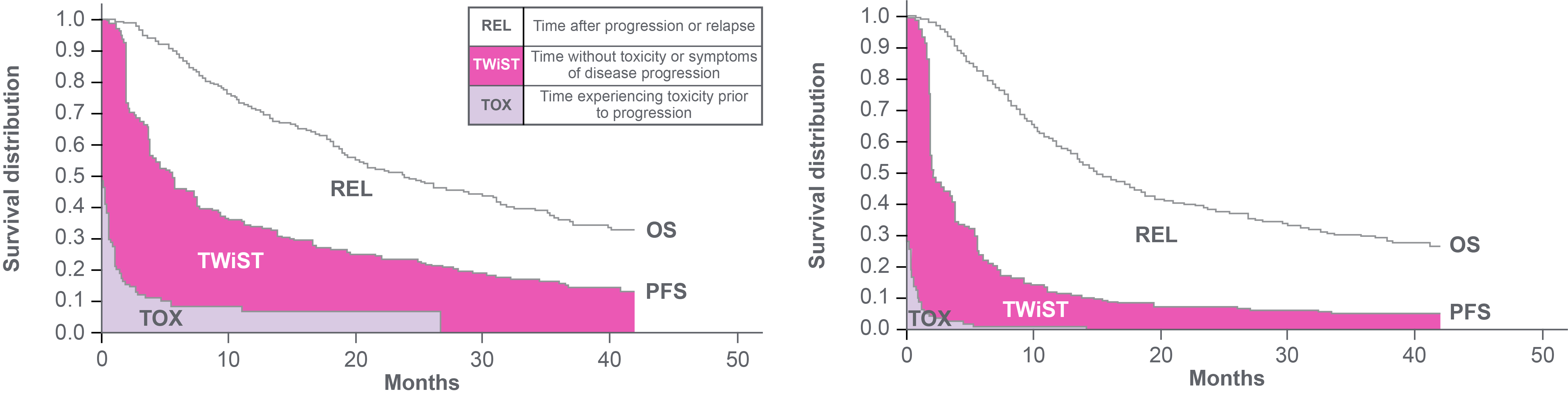
TWiST area in pink represents the improvement in �quality-adjusted survival with avelumab + BSC versus BSC alone
Data cutoff: 4 June 2021. Median follow-up: 38.0 months in the avelumab plus BSC arm and 39.6 months in the BSC alone arm.24
Q-TWiST analysis from JAVELIN Bladder 100.24
Avelumab plus BSC was associated with a 22% relative improvement in Q-TWiST vs BSC alone, exceeding the established threshold of ≥10% that indicates a clinically relevant benefit.24




Study Design


Trial Results: Efficacy


Post Hoc Analysis


Study Design


Trial Results: Efficacy


Post Hoc Analysis


Study Design


Trial Results: Efficacy


Post Hoc Analysis


We have reviewed trial data, now let's take a look at real-world data.


This includes patients who would have been ineligible for enrolment in JAVELIN Bladder 100, such as patients with an Eastern Cooperative Oncology Group (ECOG) performance status of ≥2, patients with various comorbidities, and patients who had received an alternative cisplatin-based chemotherapy regimen (such as dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin [ddMVAC]).
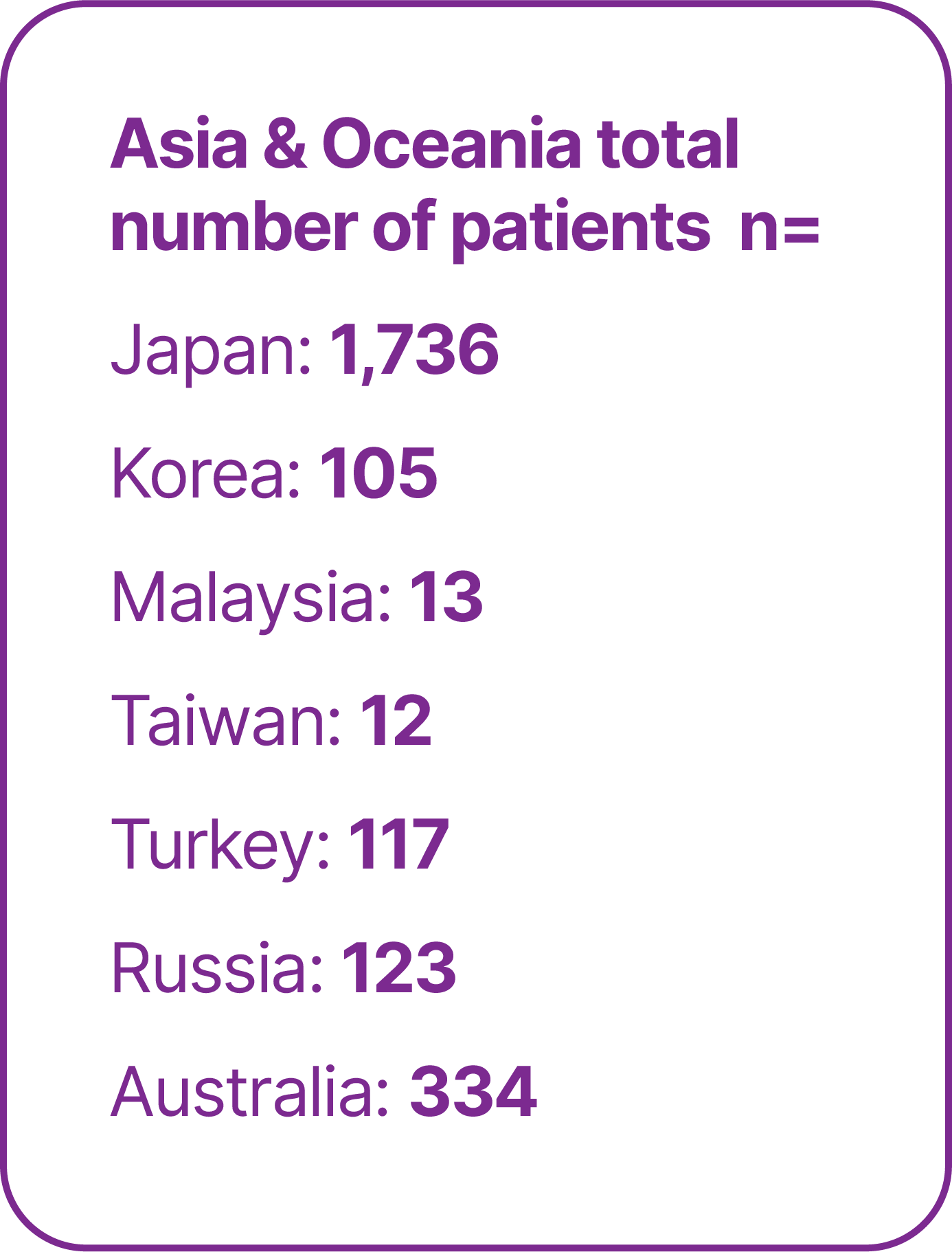
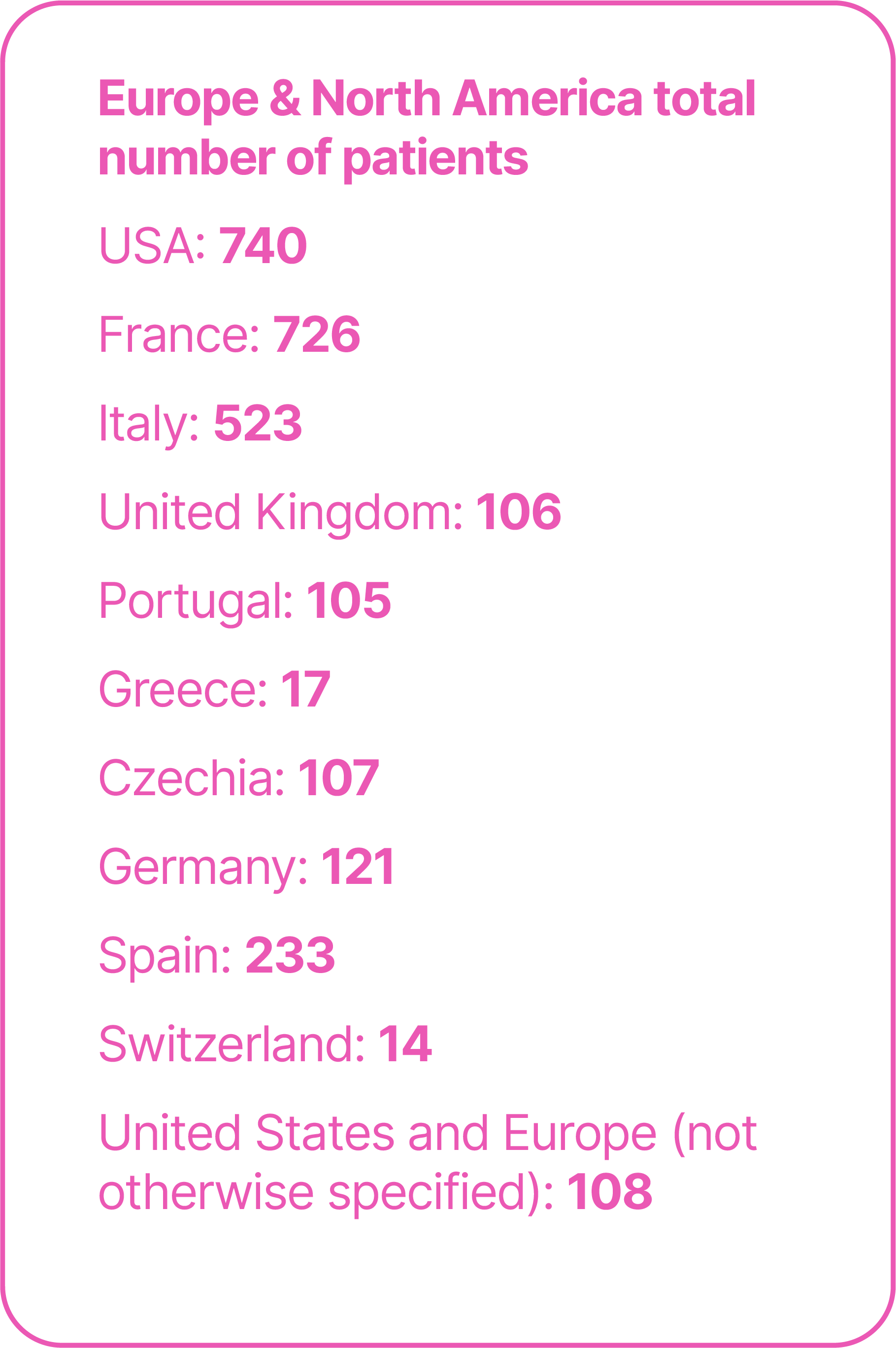

*Limitations of analyses of OS calculated from the start of 1L platinum-based chemotherapy in patients who received avelumab 1L maintenance include the following:
Analyses were performed in selected populations who remained progression free after 1L platinum-based chemotherapy;
OS includes time receiving platinum-based chemotherapy (typically 4–6 cycles) and any interval between platinum-based chemotherapy and avelumab (typically 4–10 weeks) and
OS includes immortal time bias because all patients analysed were required to have survived throughout 1L PBC to be eligible for avelumab 1L maintenance treatment.
Thus, results should be interpreted with caution.
*Overall survival calculated from the start of first-line platinum-based chemotherapy includes time receiving platinum-based chemotherapy (typically 4-6 cycles) and any treatment-free interval (typically 4–10 weeks).
Results should be interpreted with caution because of data limitations (selected population without progression after 1L PBC, immortal time bias during time receiving 1L PBC).
Abbreviations: �1L: first line; CUP, compassionate use programme; EAMS: Early Access to Medicines Scheme; OS: overall survival; PBC: platinum-based chemotherapy; PFS, progression-free survival; PMS: postmarketing surveillance; RCT: randomised controlled trial.


DEEP DIVE INTO AVENANCE: Ambispective observational study in France40
N=595
Median age: 73 years
ECOG performance status: 0 or 1 in 91%, ≥2 in 9%
Prior 1L chemotherapy�- Cisplatin plus gemcitabine in 28%�- Carboplatin plus gemcitabine in 61%�- ddMVAC in 4%
Data cutoff: December 2023
Discontinued avelumab treatment by data cutoff: 470 patients
Median duration of avelumab treatment: 5.6 months
Median OS from the start of avelumab (primary endpoint): 21.3 months
Median OS from the start of avelumab by second-line (2L) treatment (analysed in 330 patients who discontinued avelumab and received 2L treatment)�- 2L nonplatinum chemotherapy (n=163 [49% of the 2L population]): 13.6 months�- 2L platinum-based chemotherapy (n=81 [25% of the 2L population]): 16.7 months�- 2L enfortumab vedotin (n=56 [17% of the 2L population]): 36.0 months
Median PFS: 5.7 months
Median OS from the start of 1L platinum-based chemotherapy (exploratory analysis): 26.5 months* �
Avelumab-related AEs (any grade): 55% of patients, including pruritus in 10%, asthenia in 10%, and diarrhoea in 5%
N=414
Median age: 71 years
ECOG performance status: 0 or 1 in 100%
Prior 1L chemotherapy�- Cisplatin plus gemcitabine in 44%�- Carboplatin plus gemcitabine in 53%
Data cutoff: July 2023
Median duration of avelumab treatment: 3.8 months
Median OS from the start of avelumab: 26.2 months
Median PFS: 7.6 months
Median OS from the start of 1L platinum-based chemotherapy (exploratory analysis): 30.9 months*
Avelumab-related AEs (any grade): 27% of patients, including pruritus in 4%, asthenia in 3%, and fatigue in 3%
DEEP DIVE INTO READY CUP: Compassionate Use Programme in Italy43

DEEP DIVE INTO JAVEMACS: Medical chart review �study in Japan31
N=350
Median age: 73 years
ECOG performance status: 0 or 1 in 97%, ≥2 in 2%
Prior 1L chemotherapy�- Cisplatin plus gemcitabine in 56%�- Carboplatin plus gemcitabine in 33%�- ddMVAC in 9%
Data cutoff: June 2024
Discontinued avelumab treatment by data cutoff: 283 patients
Median duration of avelumab treatment: 14.3 weeks
Median OS from the start of avelumab: 31.8 months
Median OS from the start of avelumab by 2L treatment (analysed in 200 patients who discontinued avelumab and received 2L treatment):
2L enfortumab vedotin (n=133 [67% of the 2L population]): 31.8 months
2L platinum-based chemotherapy (n=41 [21% of the 2L population]): 23.5 months
2L pembrolizumab (n=17 [9% of the 2L population]): 24.3 months
Median OS from the start of 1L platinum-based chemotherapy (exploratory analysis): 38.9 months*
Survival outcomes reported in heterogeneous real-world study populations have been comparable to JAVELIN Bladder 100 Phase III trial results.


Real-world studies have also provided new insights on treatment sequencing, with median OS of up to 3 years observed in patients who received a sequence of guideline-recommended 1L platinum-based chemotherapy followed by avelumab 1L maintenance in patients without disease progression and 2L enfortumab vedotin.


Overall, real-world evidence consistently supports the use of avelumab 1L maintenance as a recommended treatment option in patients with advanced UC without progression following 1L platinum-based chemotherapy.


CONCLUSION
CONCLUSION
CONCLUSION
References
1. Saginala K et al. Epidemiology of bladder cancer. Med Sci (Basel). 2020;8(1):15.
2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer. Available at: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Last accessed 18 July 2025.
3. International Agency for Research on Cancer. GLOBOCAN 2022. Cancer Today. Available at: https://gco.iarc.fr/today/en. Last accessed 18 July 2025.
4. National Cancer Institute. SEER Cancer Stat Facts: Bladder Cancer. Available at: https://seer.cancer.gov/statfacts/html/urinb.html. Last accessed 18 July 2025.
5. Patel VG et al. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404-23.
6. Kearney M et al. Undertreatment in patients with advanced urothelial cancer: systematic literature review and meta-analysis. Future Oncol. 2024;20(16):1123-37.
7. Geynisman DM et al. Real-world treatment patterns and clinical outcomes among patients with advanced urothelial carcinoma in the United States. Urol Oncol. 2022;40(5):195.e1-195.e11.
8. Mahmoudpour SH et al. Factors associated with receipt of systemic anticancer treatment for locally advanced or metastatic urothelial carcinoma in England: a population-based study. Urol Oncol. 2024;42(12):451.e11-451 e18.
9. Niegisch G et al. Treatment patterns and clinical outcomes in metastatic urothelial carcinoma: a German retrospective real-world analysis. Future Oncol. 2024;20(19):1351-66.
10. Powles T et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-88.
11. Bamias A et al. Atezolizumab monotherapy versus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis from a randomised, controlled, phase 3 study. Lancet Oncol. 2024;25(1):46-61.
12. Grande E et al. Atezolizumab plus chemotherapy versus placebo plus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis results from a randomised, controlled, phase 3 study. Lancet Oncol. 2024;25(1):29-45.
13. Powles T et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931-45.
14. Powles T et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574-88.
15. Powles T et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol. 2024;35(6):485-90.
16. van der Heijden AG et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2025 guidelines. Eur Urol. 2025:87(5):582-600.
17. Powles T et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-92.
18. Powles T et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-30.
19. Sridhar SS et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1L chemotherapy regimen and analysis of overall survival (OS) from start of 1L chemotherapy. J Clin Oncol. 2023;DOI: 10.1200/JCO.2023.41.6_suppl.508.
20. Grivas P et al. Avelumab first-line maintenance therapy for advanced urothelial carcinoma: comprehensive clinical subgroup analyses from the JAVELIN Bladder 100 phase 3 trial. Eur Urol. 2023;84(1):95-108.
21. Gupta S et al. Avelumab first-line maintenance for advanced urothelial carcinoma: long-term outcomes from the JAVELIN Bladder 100 trial in older patients. ESMO Open. 2025;10(4):104506.
22. Aragon-Ching JB et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (aUC): long-term outcomes from the JAVELIN Bladder 100 trial in patients (pts) with high body mass index (BMI). J Clin Oncol. 2024;DOI: 10.1200/JCO.2024.42.4_suppl.600.
23. Bellmunt J et al. Avelumab first-line maintenance (1LM) for advanced urothelial carcinoma (aUC): Long-term outcomes from JAVELIN Bladder 100 in patients (pts) with low tumor burden. J Clin Oncol. 2024;DOI: 10.1200/JCO.2024.42.16_suppl.4566.
24. Grivas P et al. Avelumab First-line Maintenance for Advanced Urothelial Carcinoma: Long-term Analyses of Patient-reported Outcomes and Quality-adjusted Time Without Symptoms or Toxicity from the JAVELIN Bladder 100 Trial. Eur Urol Oncol. 2025;S2588-9311(25)00098-7.


bladder
ureter
renal pelvis
urethra
25. Grivas P et al. Avelumab first-line maintenance for locally advanced or metastatic urothelial carcinoma: results from the real-world US PATRIOT-II study. Clin Genitourin Cancer. 2024;22(6):102238.
26. Moon HH et al. Real-world treatment patterns, sequencing, and outcomes in patients with locally advanced or metastatic urothelial carcinoma receiving avelumab first-line maintenance in the United States. Curr Oncol. 2024;31(9):5662-76.
27. Carson KR et al. Real-world (rw) treatment (tx) patterns, sequencing, and outcomes in US patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC) treated with avelumab first-line maintenance (1LM). J Clin Oncol. 2024;DOI: 10.1200/JCO.2024.42.16_suppl.e16621.
28. Sura S et al. Real-world clinical outcomes with first-line systemic treatment and avelumab maintenance in US patients with locally advanced or metastatic urothelial carcinoma: the SPEAR Bladder-II study. Curr Oncol. 2025;32(4):187.
29. Kikuchi E et al. J-AVENUE: A retrospective, real-world study evaluating patient characteristics and outcomes in patients with advanced urothelial carcinoma treated with avelumab first-line maintenance therapy in Japan. Int J Urol. 2024;31(8):859-67.
30. Kobayashi T et al. Evaluation of avelumab first-line (1L) maintenance therapy and subsequent treatment (tx) in patients (pts) with locally advanced or metastatic urothelial carcinoma (la/mUC) using a large claims database in Japan: JAVEMACS-D. J Clin Oncol 2025;DOI: 10.1200/JCO.2025.43.5_suppl.700.
31. Kitamura H et al. Real-world effectiveness and treatment (tx) patterns in patients (pts) with locally advanced/metastatic urothelial carcinoma (la/mUC) receiving avelumab first-line maintenance (1LM) in Japan: results from the JAVEMACS chart review study. J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.701.
32. Kikuchi E et al. Primary analysis of post-marketing surveillance (PMS) data for avelumab maintenance therapy in patients (pts) with curatively unresectable urothelial carcinoma (UC) in Japan. Ann Oncol. 2024;35(Suppl 2):S1155.
33. Minato A et al. Clinical outcomes of enfortumab vedotin in advanced urothelial carcinoma with prior avelumab versus pembrolizumab therapy. Anticancer Res. 2024;44(8):3419-26.
34. Taneda Y et al. Clinical outcomes and treatment patterns of maintenance avelumab in locally advanced or metastatic urothelial carcinoma: a multicenter collaborative study. Jpn J Clin Oncol. 2025;55(5):522-30.
35. Park SH et al. Avelumab first-line maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma: real-world results from a Korean expanded access program. Front Oncol. 2024;14:1403120.
36. Park SH et al. Interim analysis (IA) of SPADE: A prospective, real-world study of avelumab first-line maintenance (1LM) treatment in patients (pts) with locally advanced or metastatic urothelial carcinoma (la/mUC) in the Asia-Pacific (APAC) region. Ann Oncol. 2024;35:S1509-9.
37. Ismail FB et al. Avelumab first-line maintenance for advanced urothelial carcinoma: real-world results from the early access program in Malaysia. Abstract A-0177. ASCOMOS Annual Meeting, 11-13 October 2024.
38. Inderjeeth A-J, Wakeling S, O’Haire S, et al. Real-world avelumab uptake for first-line (1L) treatment for metastatic urothelial bladder cancer (mUC). Asia Pac J Clin Oncol 2024;20(S1):Abstract 58.
39. Moujaber T, Tran B, Liow E, et al. Real-world data from early access and patient access programs of avelumab first-line maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma in Australia. Asia Pac J Clin Oncol 2023;19(S2):Abstract 47.
40. Barthelemy P et al. Real-world study of avelumab first-line maintenance treatment in patients with advanced urothelial carcinoma in France: overall results from the noninterventional AVENANCE study and analysis of outcomes by second-line treatment. Eur Urol Oncol. 2025;8(2):407-16.
41. Belrhali I. Real-world data of avelumab first-line maintenance treatment in patients with advanced urothelial carcinoma: Experience French. Eur Urol Open Sci. 2024;69(Suppl 2):312.
42. Moinard-Butot F et al. Treatment sequence (TS) and overall survival (OS) in patients with metastatic urothelial cancer (mUC): An observational, multicenter, real-life STATES-Bladder study. J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.724
43. Antonuzzo L et al. READY: REAl-world Data from an Italian compassionate use program of avelumab first-line maintenance (1LM) for locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol. 2023;DOI: 10.1200/JCO.2023.41.6_suppl.469.


44. Gambale E et al. Neutrophil-to-eosinophil ratio predicts the efficacy of avelumab in patients with advanced urothelial carcinoma enrolled in the MALVA study (Meet-URO 25). Clin �Genitourin Cancer. 2024;22(4):102099.
45. Jones RJ et al. Avelumab first-line (1L) maintenance therapy in advanced urothelial carcinoma: Final analysis from a real-world study in the UK. J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.723.
46. Gonçalves L et al. Avelumab maintenance therapy in advanced urothelial carcinoma: implications of timing and treatment sequencing. Cancers (Basel). 2025;17(5):898.
47. Tsimafeyeu I et al. Maintenance therapy with avelumab for patients with metastatic urothelial carcinoma: a real-world, ambispective RAVE-Bladder study. Cancer Med. 2025;14(4):e70636.
48. Alevizopoulos N et al. Avelumab as maintenance treatment in advanced urothelial cancer. A single oncological centre’s experience. Eur Urol Open Sci. 2023;57(Suppl 1):S317.
49. Studentova H et al. Avelumab first-line (1L) maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma (la/mUC) in the Czech Republic: Updated real-world results from a national reimbursement registry. J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.693.
50. Tural D et al. Avelumab maintenance in patients with metastatic urothelial carcinoma in a real-life expanded-access program (EAP). J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.708.
51. Goebell PJ et al. Real-world effectiveness and safety of avelumab first-line maintenance (1LM) treatment in patients with locally advanced or metastatic urothelial carcinoma (la/mUC): Second interim analysis of the AVENUE study. J Clin Oncol. 2025;DOI: 10.1200/JCO.2025.43.5_suppl.706.
52. Schlack K et al. Real-world treatment patterns and clinical outcomes in patients with locally advanced or metastatic urothelial carcinoma in Germany: retrospective CONVINCE study. J Cancer Res Clin Oncol. 2025;151(3):100.
53. Sotelo M et al. Real-world data (RWD) with avelumab in patients (pts) with locally advanced or metastatic urothelial cancer (la-mUC): The AVEBLADDER study. Abstract e16559. J Clin Oncol. 2024;DOI: 10.1200/JCO.2024.42.16_suppl.e16559.
54. Juan Fita MJ et al. SOGUG-AVELUMAB_RWD: Comprehensive analysis of real-word data to evaluate the effectiveness and safety of avelumab maintenance therapy in patients with stage IV urothelial carcinoma conducted in Spain. Abstract e16561. J Clin Oncol 2024;42(Suppl 16).
55. Bakaloudi DR et al. Response and outcomes of maintenance avelumab after platinum-based chemotherapy (PBC) in patients with advanced urothelial carcinoma (aUC): "real world" experience. Clin Genitourin Cancer. 2023;21(5):584-93.
56. Maráz A et al. Nationwide study of real-world treatment patterns and clinical outcomes in patients with metastatic urothelial carcinoma in Hungary. Adv Ther. 2023;40(12):5475-88.
57. Kearney M et al. Use of inpatient systemic chemotherapy and/or radiotherapy and related predictive factors, healthcare resource utilization, and direct hospitalization costs for metastatic urothelial cancer: findings from a real-world retrospective observational study derived from the national hospital discharge claims database in Italy. BMC Cancer. 2024;24(1):1470.






























Adverse events should be reported. Reporting forms and information can be found via: Great Britain & Northern Ireland – The Yellow Card Scheme at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App store; Ireland – HPRA Pharmacovigilance at www.hpra.ie. ��Adverse events should also be reported to Merck for the UK via email (medinfo.uk@merckgroup.com) or telephone (0208 818 7373) and Republic of Ireland (medinfo.uk@merckgroup.com) (1800 719881).
Prescribing Information can be found here
Data from JAVELIN Bladder 100 also showed that avelumab 1L maintenance plus BSC improved quality-adjusted survival compared with BSC alone.
Oncology and Urology

