Exploring TYK2 Inhibition in
Dermatology

Moderate-to-Severe Plaque Psoriasis


The publication of this �infographic was supported by �Bristol Myers Squibb.
This content is intended for �US healthcare professionals only.
U.S. Prescribing Information
Kinase Overview
Tyrosine kinase 2 (TYK2) is an intracellular enzyme involved in the relay of immune signals initiated by specific cytokines, including IL-23, IL-12, and Type I IFN.
ACTIVE�(ATP-binding)�domain:
SIMILAR�across family�members3




REGULATORY�(pseudokinase) domain:
DISTINCT�across family�members3
TYK2 and JAK1/2/3 are members of the shared JAK family of protein kinases, but are structually distinct from each other.1,2








TYK2
JAK1
JAK2
JAK3
The JAK Family
Systems affected by different cytokines mediated through JAK1/2/3 and TYK2
TYK2 and JAK1/2/3 work in pairs to mediate signals of different cytokines, resulting in different downstream effects4-12
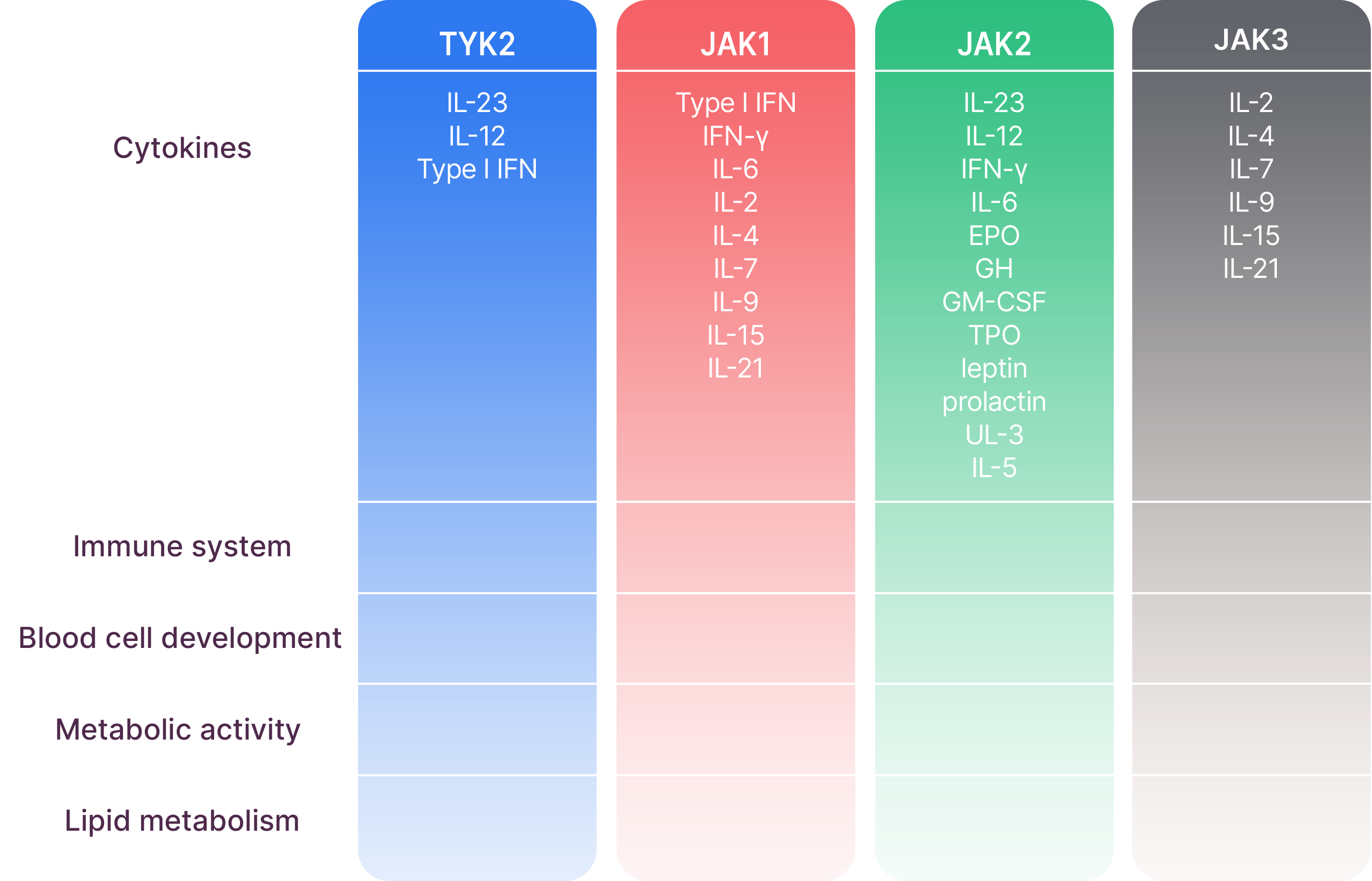
This list of systems affected by the different TYK2 and JAK1/2/3 pairings and associated cytokines is not exhaustive. Certain cytokines might also be modulated by JAK and TYK2 trimers.

Pathogenesis of psoriasis
Psoriasis is a complex, chronic, systemic condition driven in part by the IL-23/IL-17 axis.
Type I IFN, IL-23, and IL-17 play critical roles in the pro-inflammatory �signaling pathways that lead to the development of psoriasis13-15
IL-23/Th17 axis in psoriasis
IL-2315-19
TYK2
JAK2
IL-17
IL-17 production is mediated by IL-23. �Both are key inflammatory cytokines in psoriasis
e.g.,Th1715


Disclaimers

Deucravacitinib reduced psoriasis-associated gene expression in psoriatic skin in a dose-dependent manner,�including reductions in IL-23 pathway- and Type I IFN-regulated genes.
Deucravacitinib reduced IL-17A, IL-19, and beta-defensin by 47-50%, 72%, and 81-84%,�respectively, following 16 weeks of once-daily treatment.
The relationship between these pharmacodynamic markers and the mechanism(s) by which�deucravacitinib exerts its clinical effects is unknown.
TYK2 is a member of the Janus kinase family.25



Deucravacitinib is a first-in-class, highly selective, allosteric, oral TYK2 inhibitor.25,26
Deucravacitinib is a TYK2 inhibitor indicated for the�treatment of adults with moderate-to-severe plaque�psoriasis who are candidates for systemic therapy �or phototherapy.
Limitations of Use:�Deucravacitinib is not recommended for use in combination with other potent immunosuppressants.
Deucravacitinib is associated with the following Warnings and Precautions: Hypersensitivity, Infections, Tuberculosis, Malignancy including Lymphomas, Rhabdomyolysis and Elevated CPK, Laboratory Abnormalities, Immunizations, and Potential Risks Related to JAK inhibition. Please see additional Important Safety Information for deucravacitinib below.


Patient experience in clinical trials and the post-marketing setting
5,046.7 PY of total exposure through 5 years of PsO clinical trials
From POETYK PSO-1, PSO-2 and through LTE data cutoff27-32
Median duration of exposure was 4.2 years.30�Approximately 30% of patients had more than 5 years of exposure.30
Completed pivotal Phase III clinical trials in PsO27,28
2
Ongoing pivotal Phase III LTE trial in PsO29,30
1
Up to 15,300 patient-years in clinical trials and the US �post-marketing setting31,32



Click here to view the Study Design

Safety
Pooled safety data through Week 16 for PSO-1 and PSO-225
Adverse reactions that occurred in ≥1% of subjects with plaque psoriasis in the deucravacitinib group and more frequently than in the placebo group in trials PSO-1 and PSO-2 through Week 16
Adverse reactions that occurred in <1% of�patients in the deucravacitinib group were�herpes zoster
The incidence of serious infections were�reported in 5 patients (2.0/100 PY) treated�with deucravacitinib and 2 patients �(1.6/100 PY) treated with placebo in the �16-week period
The most common serious infections�reported during the 52-week period were�pneumonia and COVID-19
Malignancies (excluding NMSC) through�Week 52 (total exposure of 986 PY with�deucravacitinib) were reported in 3 patients�treated with deucravacitinib (0.3/100 PY)
During clinical trials, including an open-label�extension trial, 3 deucravacitinib patients�(0.1/100 PY) developed lymphoma
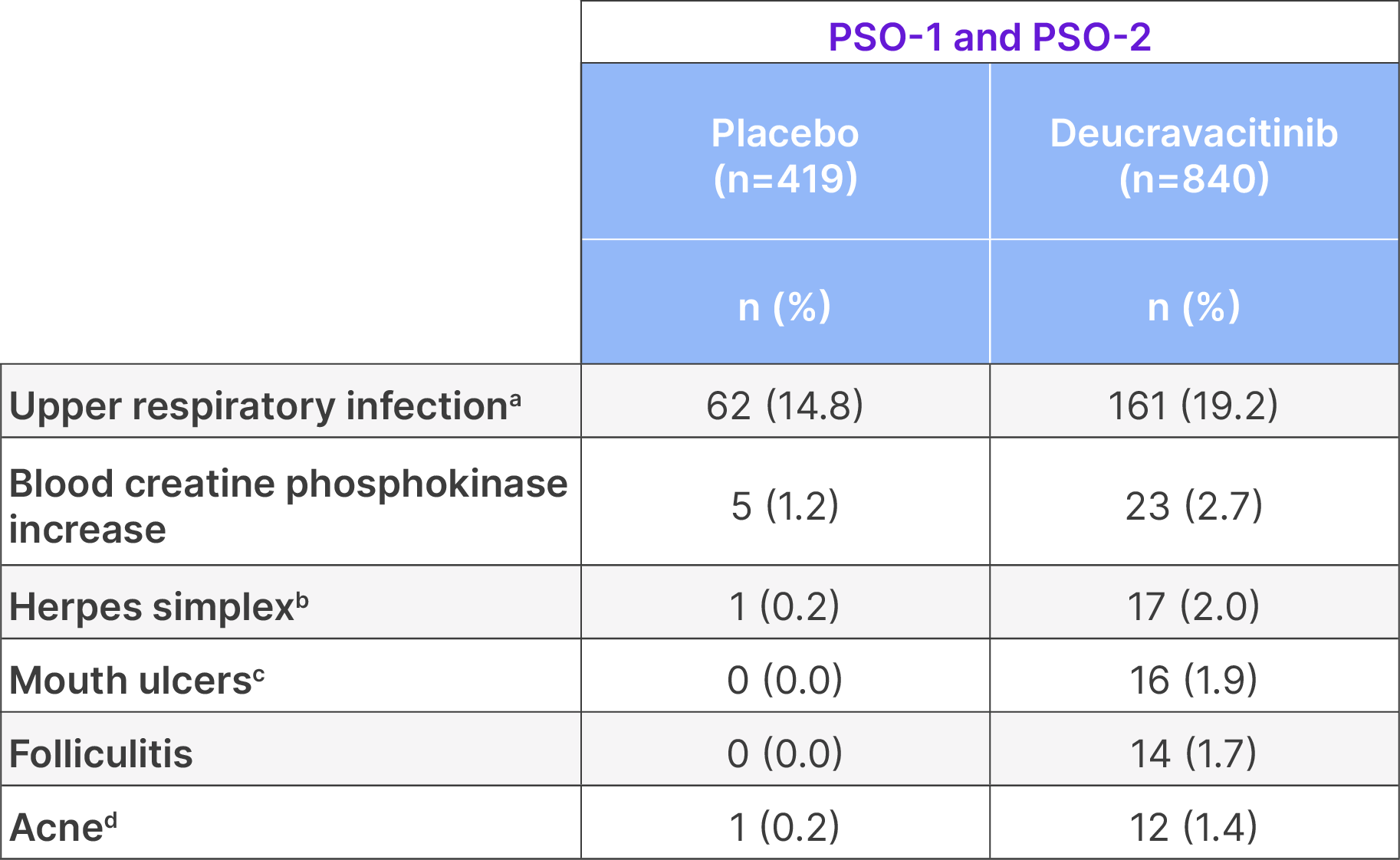
aIncludes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and �unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal). �bIncludes oral herpes, genital herpes, herpes simplex, and herpes virus infection. cIncludes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis. dIncludes acne, acne cystic, and dermatitis acneiform.

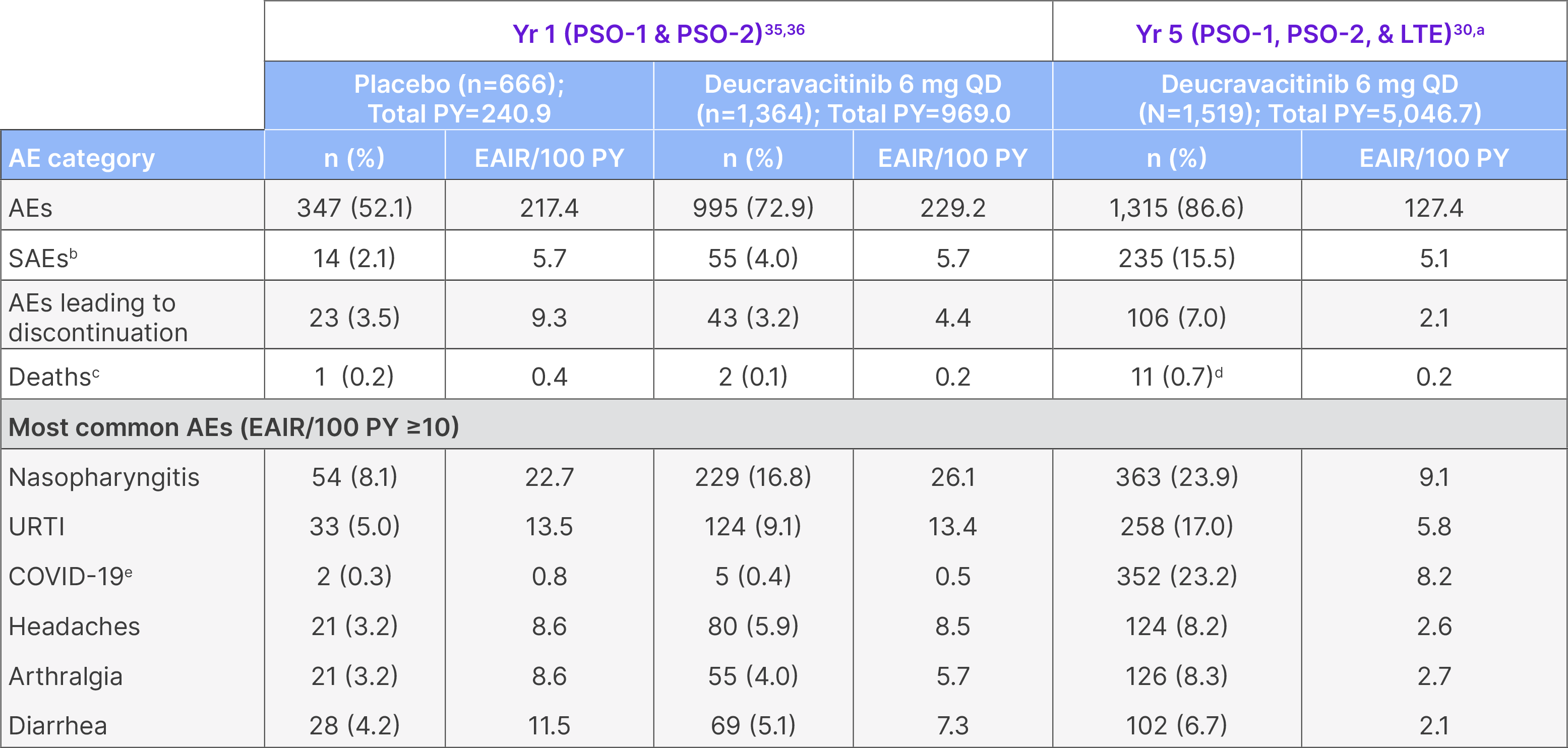
Year 5: Overall safety summary
AE: adverse event; EAIR: exposure-adjusted incidence rate; PY: patient-years; �QD: once daily; SAE: serious adverse event; URTI: upper respiratory tract infection.
Not all patients were receiving deucravacitinib 6 mg QD continuously throughout this period. aThis represents the pooled POETYK PSO-1, PSO-2, and �LTE population through the cutoff date of September 2, 2024. 79.4% of patients had total deucravacitinib exposure for ≥12 months, and 29.6% of �patients had deucravacitinib exposure for ≥60 months. bSerious AEs through Year 3: n=167/1,519; EAIR/100 PY=5.5 (total PY=3,294.3). Serious AEs �through Year 4: n=205/1,519; EAIR/100 PY=5.0 (total PY=4,392.8). cIn POETYK PSO-1 and PSO-2 through 1 year, 1 patient discontinued deucravacitinib �after 4 days of treatment due to prohibited medication (leflunomide) and died 9 days later due to heart failure and sepsis. Another death occurred �between Weeks 16 and 52 and was due to hepatocellular carcinoma in a patient with a history of hepatitis C virus infection and liver cirrhosis. After �Week 52, 6 deaths were due to COVID-19, and 1 was due to a ruptured aortic aneurysm in a patient with cardiovascular risk factors. One patient died �due to an unknown cause >30 days after discontinuing treatment but was originally included in the 2-year data; this patient was not included in the 3-, �4- or 5-year data. dAfter Week 52, 7 deaths were due to COVID-19 (2 deaths were considered related to treatment by the investigator, and the other 5 �deaths were considered unrelated to treatment by the investigator). One patient with cardiovascular risk factors died due to a ruptured aortic �aneurysm, which was considered unrelated to treatment by the investigator. One patient with a history of Type 2 diabetes mellitus with neuropathy, hypertension, and hypercholesterolemia died due to sudden death of unknown cause, which was not considered related to treatment by the �investigator. ePOETYK PSO-1, PSO-2, and LTE trials were conducted in part during the COVID-19 pandemic. COVID-19 through Year 3: n=242/1,519; �EAIR/100 PY=8.0 (total PY=3,294.3). COVID-19 through Year 4: n=321/1,519, EAIR/100 PY=8.3 (Total PY=4,392.8).
View the Study Design




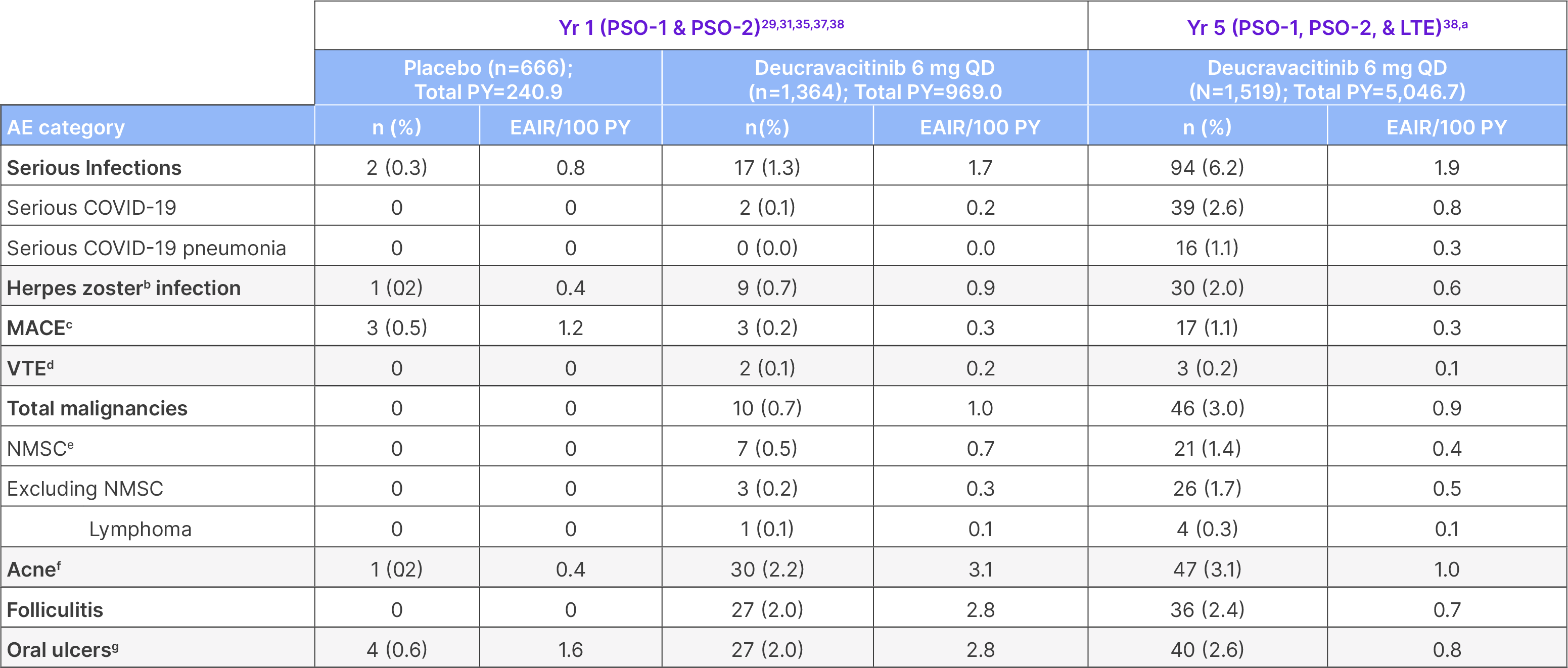
Year 5: Adverse events of interest
EAIR: exposure-adjusted incidence rate; MACE: major adverse cardiovascular events; NMSC: non-melanoma skin cancer; PY: patient-years; QD: once daily; VTE: venous thromboembolism.
Not all patients were receiving deucravacitinib 6 mg QD continuously throughout this period. aThis represents the pooled POETYK PSO-1, PSO-2, and �LTE population through the cutoff date of September 2, 2024. 79.4% of patients had total deucravacitinib exposure for ≥12 months, and 29.6% of �patients had deucravacitinib exposure for ≥60 months. bOne patient who was coded as having herpes zoster had corneal/ocular disease related to �herpes virus infection diagnosed by an ophthalmologist with a positive qualitative chickenpox virus antigen (epithelial cells). One patient who was �coded as having ophthalmic herpes zoster with swelling of the eyelids was referred for ophthalmology consultation, which was noted as normal; there �was no corneal/ocular disease related to herpes virus infection. cMACE were adjudicated and were defined as non-fatal stroke, non-fatal myocardial infarction, or cardiovascular death. dVTE was defined as deep vein thrombosis and pulmonary embolism. eIncludes preferred terms of squamous cell carcinoma, squamous cell carcinoma of the skin, and Bowen’s disease. fIncludes acne, acne cystic, and dermatitis acneiform. gMouth ulcers include �mouth ulceration, aphthous ulcers, tongue ulceration, and stomatitis.
View the Study Design

Efficacy
In POETYK PSO-1 and PSO-2, deucravacitinib had proven superior response rates �across PASI 75 and sPGA 0/1 co-primary endpoints vs placebo at Week 1626-28,31,39
PASI 75 Response Rate (NRI)
sPGA 0/1 Response Rate (NRI)
Significantly greater proportions of patients in the deucravacitinib arms�versus placebo arms (P<0.0001) achieved both a PASI 75 and�an sPGA 0/1 response at Week 16 in both trials25,32,39
View the Study Design


PSO-125,32
PSO-225,39


13
58
53
9

PSO-125,32
PSO-225,39

7
54
50
9




Co-primary endpoints at Week 1625,27,32,39:
PASI 75 for deucravacitinib vs placebo: PSO-1: 58% (n=193/330) vs 13% (n=21/166),�P<0.0001; PSO-2: 53% (n=271/511) vs 9% (n=24/255), P<0.0001
sPGA 0/1 for deucravacitinib vs placebo: PSO-1: 54% (n=178/330) vs 7% (n=12/166),�P<0.0001; PSO-2: 50% (n=253/511) vs 9% (n=22/255), P<0.0001

Select secondary endpoints�at Week 2425-28,32,39:

POETYK PSO-LTE data�at 256 weeks25-28,30,32:
Key Learnings

Abbreviations

References

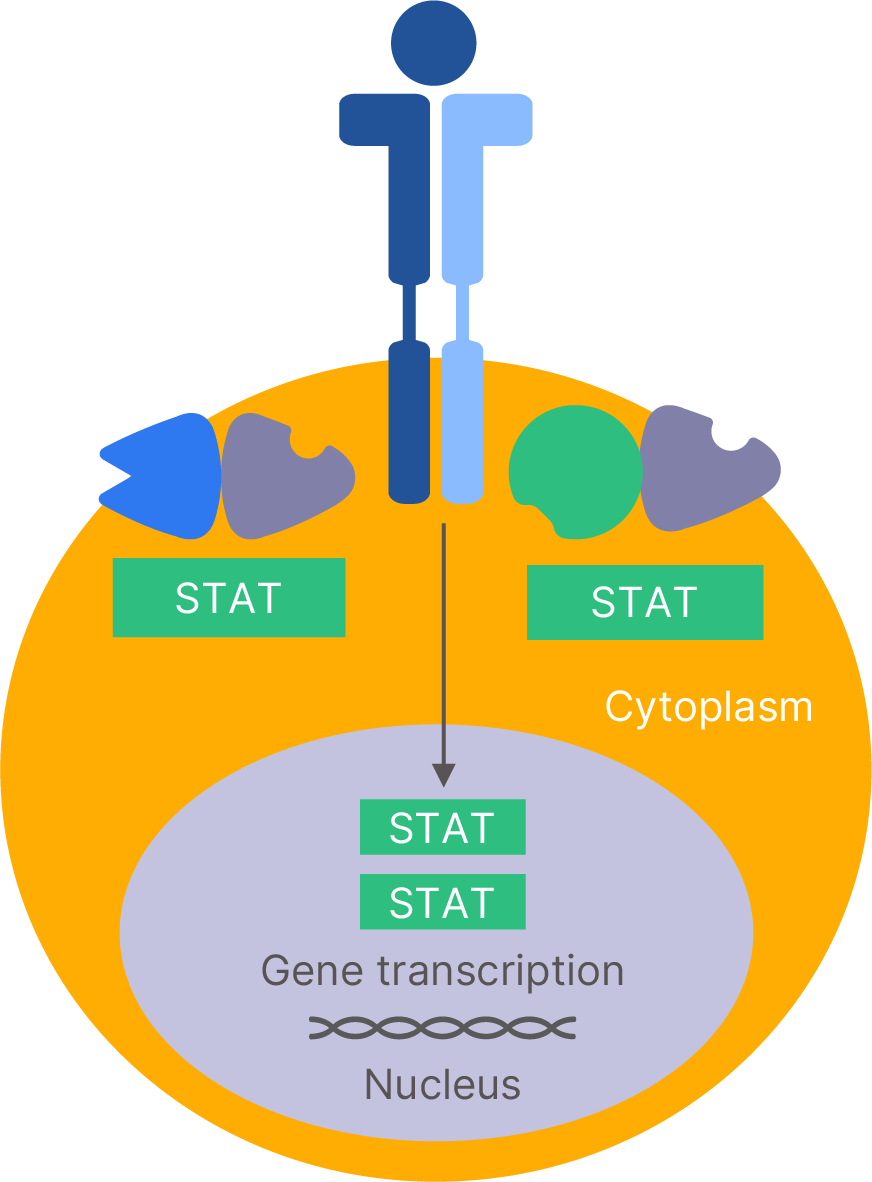
Deucravacitinib inhibits TYK2 implicated in �the pathogenesis of psoriasis
Deucravacitinib (SOTYKTU®) selectively binds to �the regulatory domain of TYK215,20,21
Deucravacitinib
Extracellular
(inactive state)
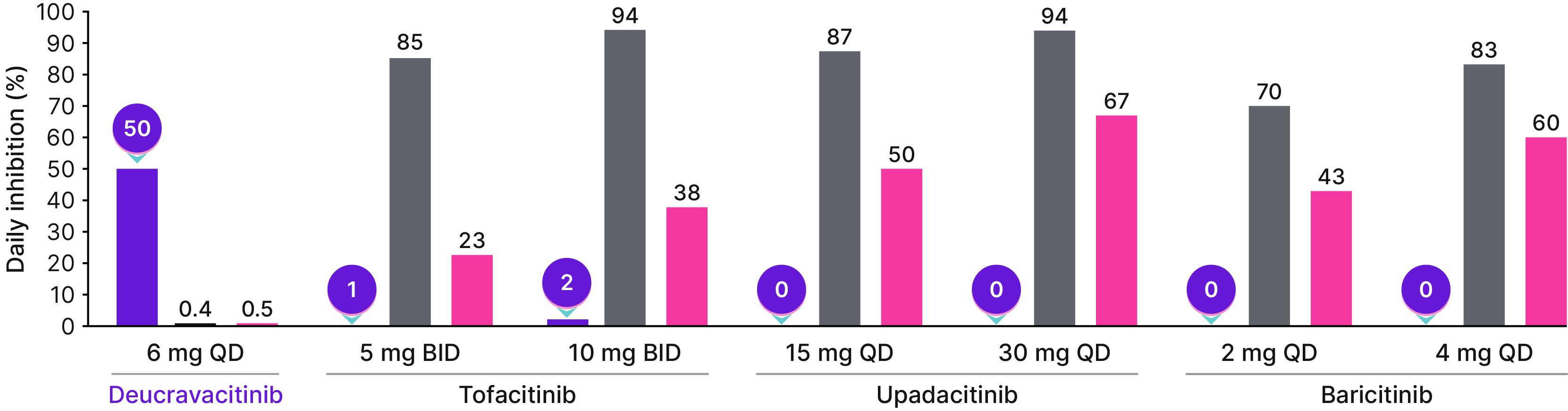
At clinically relevant doses in in vitro assays, tofacitinib, upadacitinib, and baricitinib demonstrate varying degrees of JAK 1/2/3 and ≤2% TYK2 daily percent inhibition25,26†‡
Daily percent inhibition in whole blood assays by deucravacitinib and JAK inhibitors
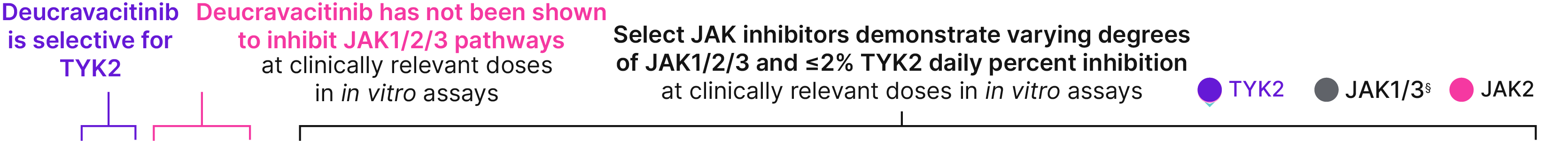
Selectivity data do not imply comparable indications, efficacy, safety, or interchangeability. �JAK inhibitors are not indicated for patients with moderate-to-severe plaque psoriasis.
*Not all FDA approved doses of the JAK inhibitors were included in this analysis.�†Simulated daily average percent inhibition of TYK2 and JAK 1/2/3 signaling was calculated using an equation based on the average drug concentration, whole blood IC50 value, and the Hill coefficient.�‡The following were used to measure pathway inhibition: JAK1/3, IL-2-induced STAT5 phosphorylation; JAK2, TPO-induced STAT3 phosphorylation; TYK2, IL-12-induced IFN-ɣ production.��§JAK1/3 are grouped together due to IL-2–induced STAT5 phosphorylation in in vitro whole-blood assays.25
SELECT IMPORTANT SAFETY INFORMATION
Potential Risks Related to JAK Inhibition: It is not known whether TYK2 inhibition may be associated with the observed or potential adverse reactions of JAK inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor... Read more


SELECT IMPORTANT SAFETY INFORMATION
Potential Risks Related to JAK Inhibition: It is not known whether TYK2 inhibition may be associated with the observed or�potential adverse reactions of JAK inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid�arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality,�including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis,�pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with �the JAK inhibitor compared to those treated with TNF blockers. Deucravacitinib is not approved for use in RA.
Please see additional Important Safety Information below.

Deucravacitinib clinical trial exposure of ~5,000 PY based on pooled POETYK PSO-1, PSO-2, and PSO-LTE populations exposed to deucravacitinib (N=1,519) through September 2, 2024.25,30
Exposure to deucravacitinib in the US post-marketing setting estimated to be ~10,200 PY based on number of prescriptions dispensed, which is estimated to be 121,400 Rxs*† from ~9,600 prescribers.25,30*
*Based on Symphony METYS TRx data and BMS Bridge Dispense data, measured by ICD-10 code L40.0. ICD-10 code does not specify severity. Assumes that patients were prescribed deucravacitinib as indicated in the prescribing information and had moderate-to-severe plaque psoriasis. The date coverage period is 9/2022 through 12/31/24. Numbers have been rounded.
†Includes new patients and refills.


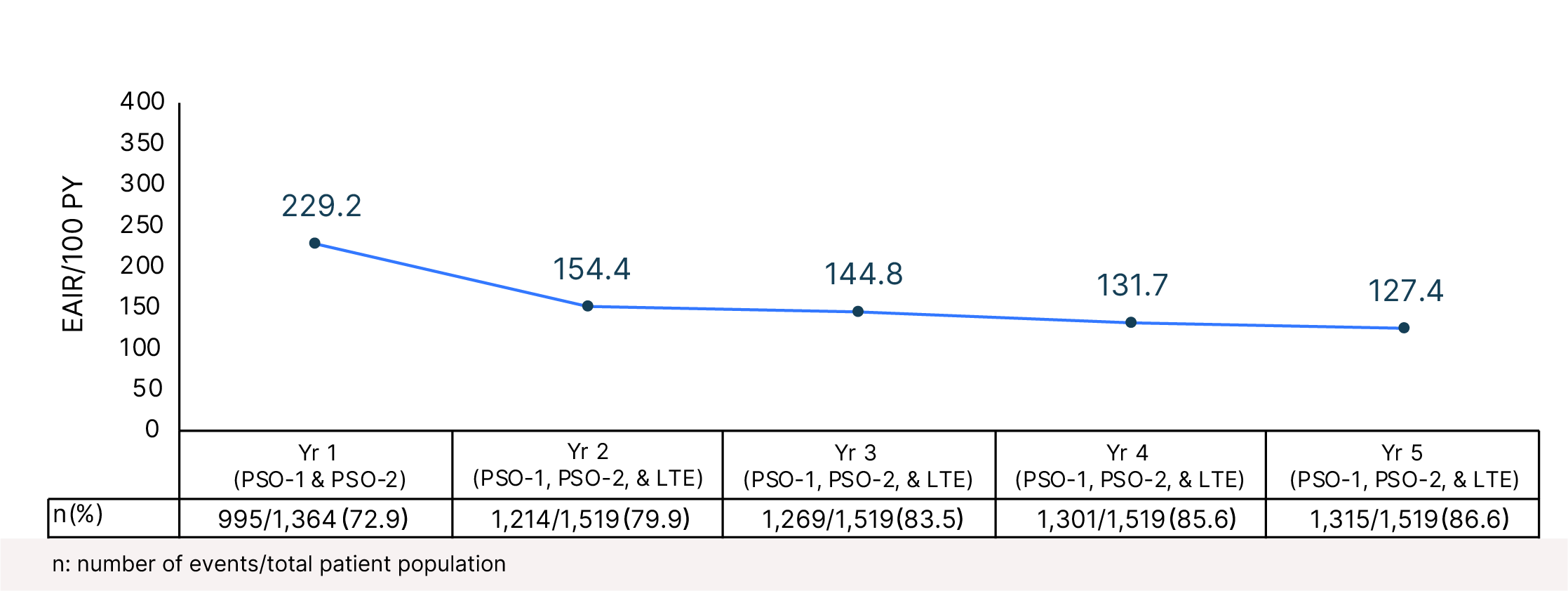
Cumulative Year-by-Year EAIRs �for AEs (as-treated population)
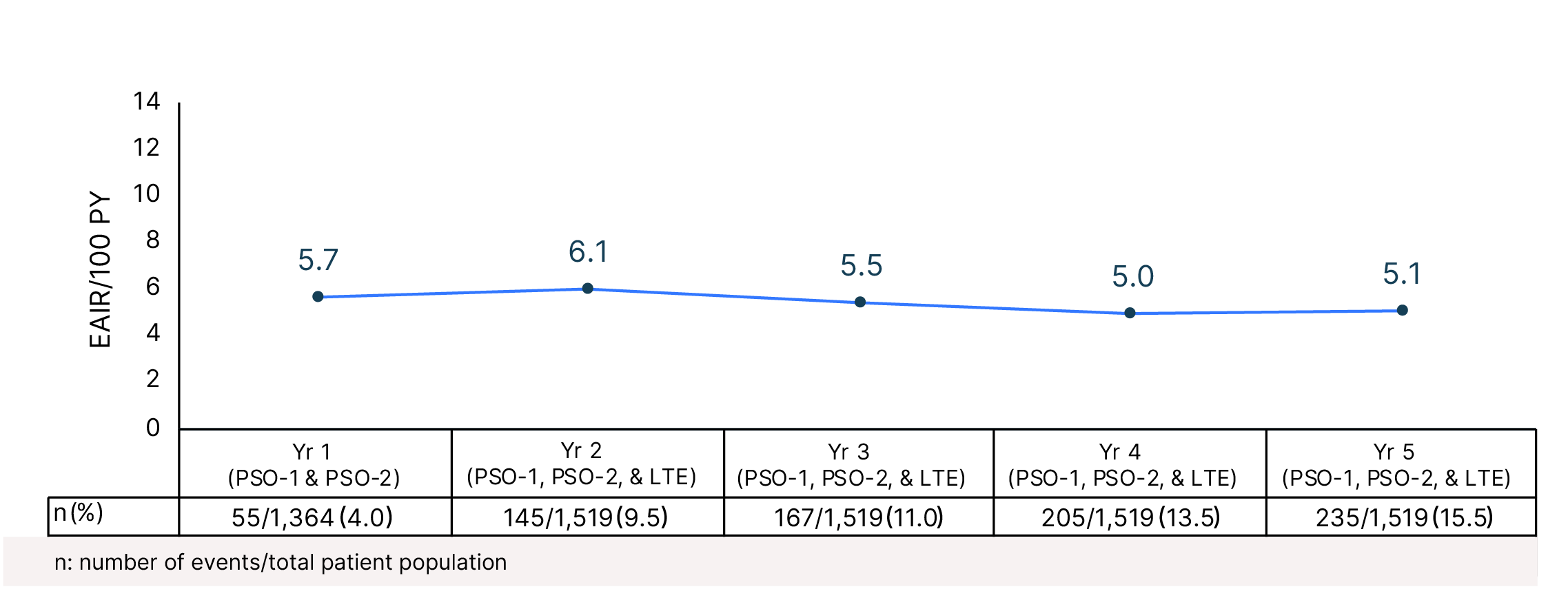

Cumulative Year-by-Year EAIRs for �SAEs (as-treated population)









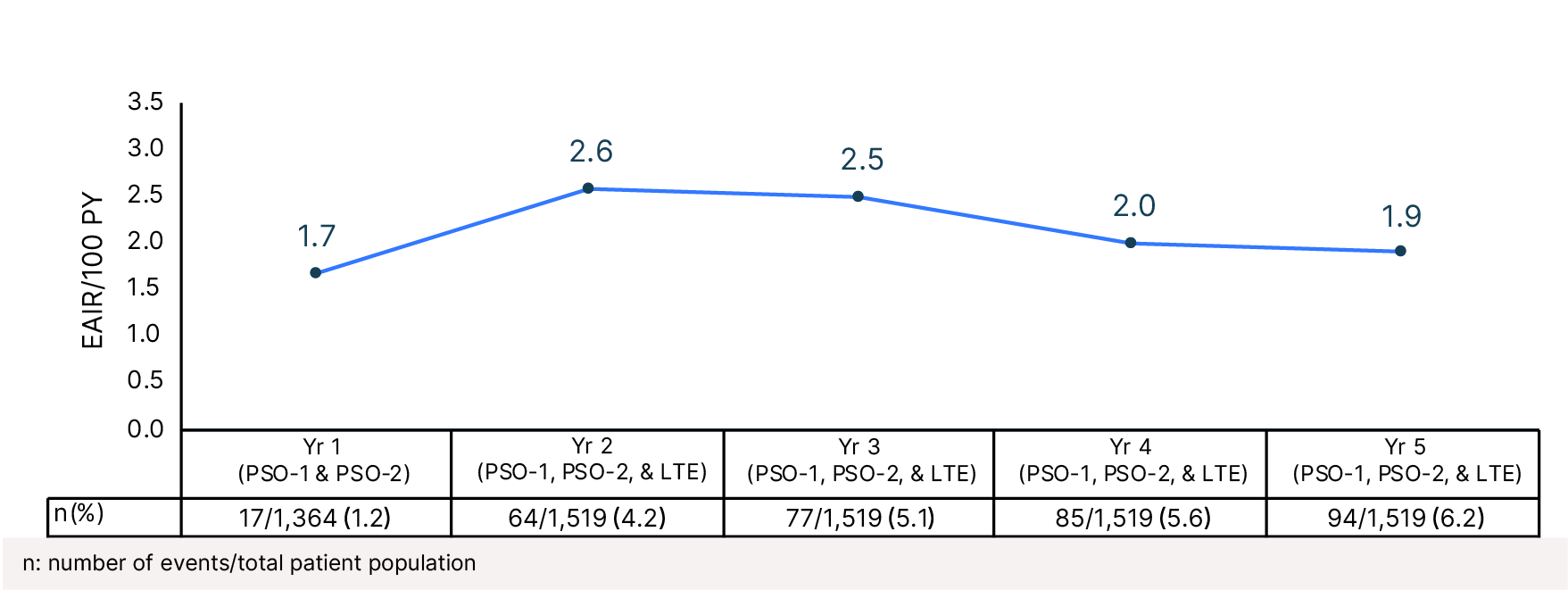
Cumulative Year-by-Year EAIRs for Serious Infections (as-treated population)29,31,35,37,38
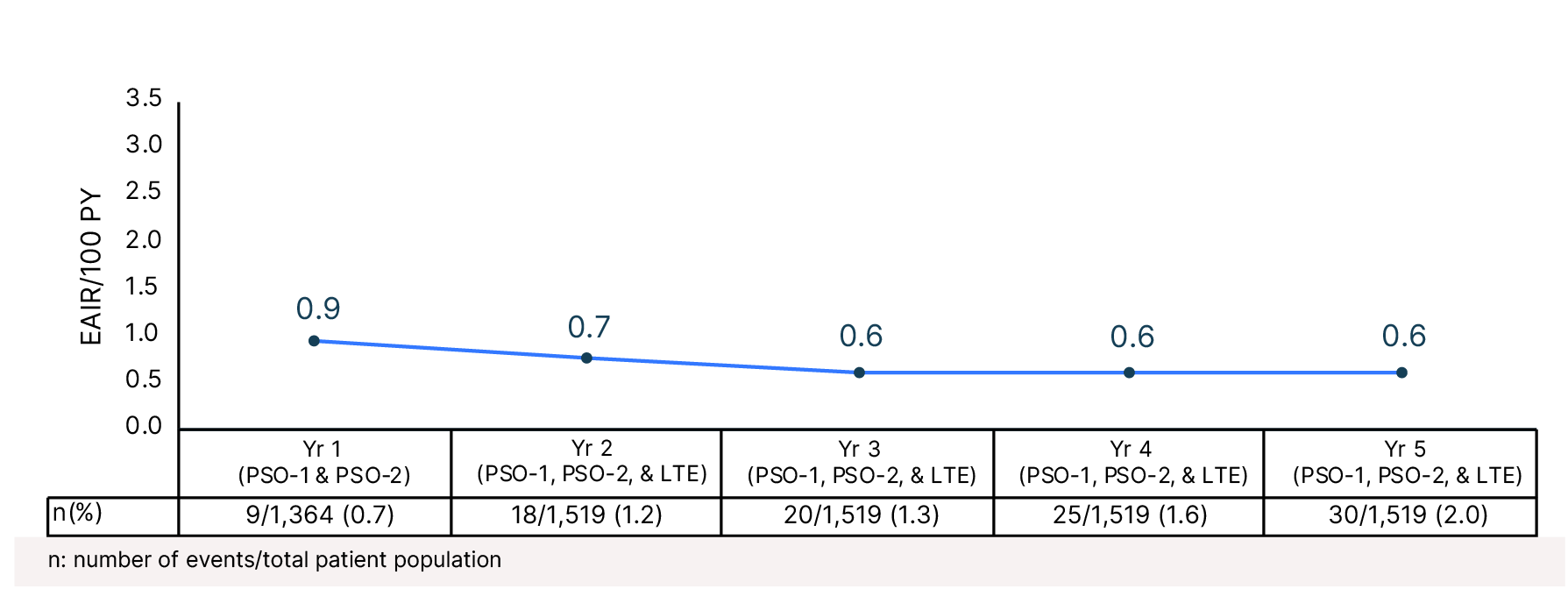

Cumulative Year-by-Year EAIRs for Herpes Zoster Infections (as-treated population)29,31,35,37,38
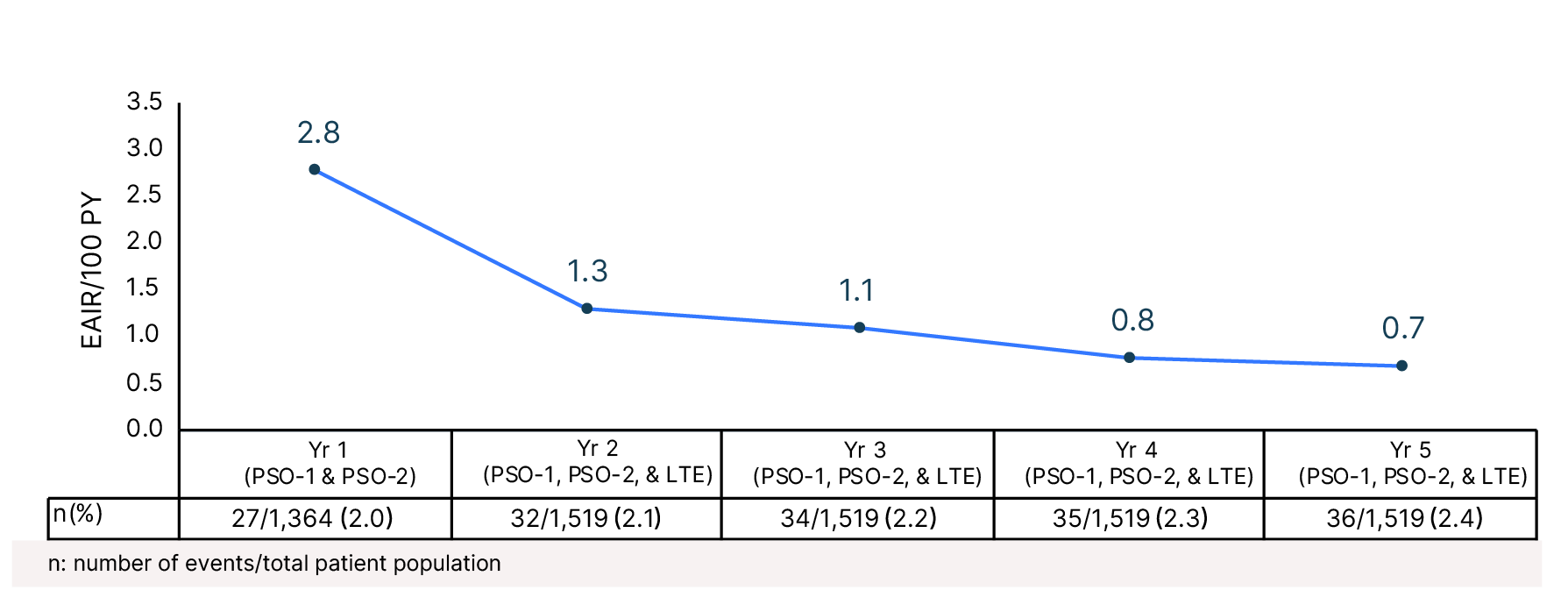

Cumulative Year-by-Year EAIRs for Folliculitis�(as-treated population)29,31,35,37,38
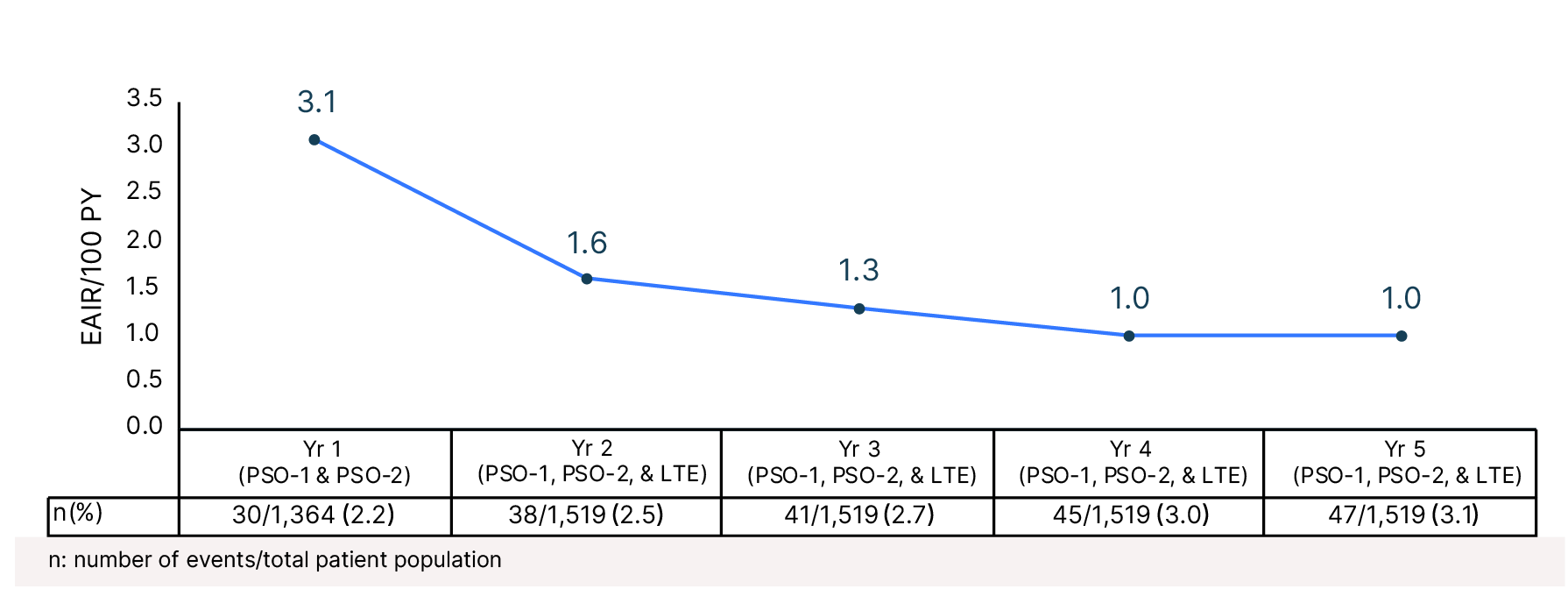

Cumulative Year-by-Year EAIRs for Acne �(as-treated population)29,31,35,37,38
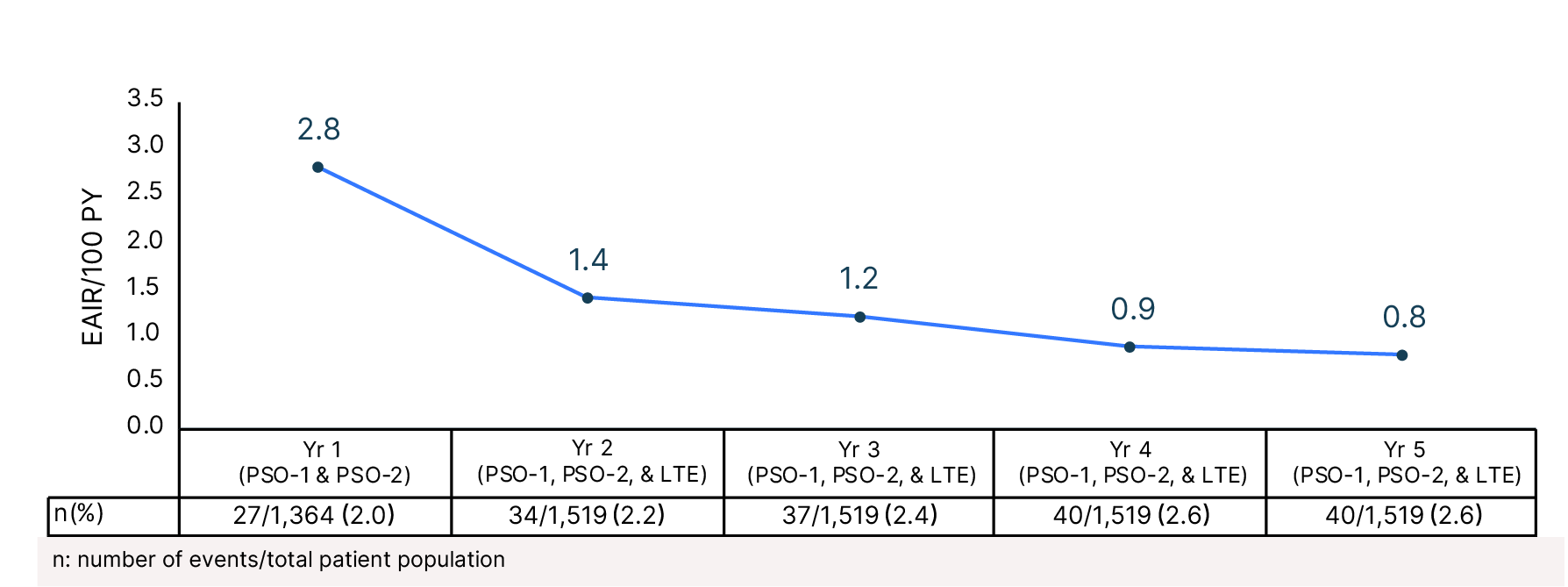

Cumulative Year-by-Year EAIRs for �Oral Ulcers (as-treated population)29,31,35,37,38
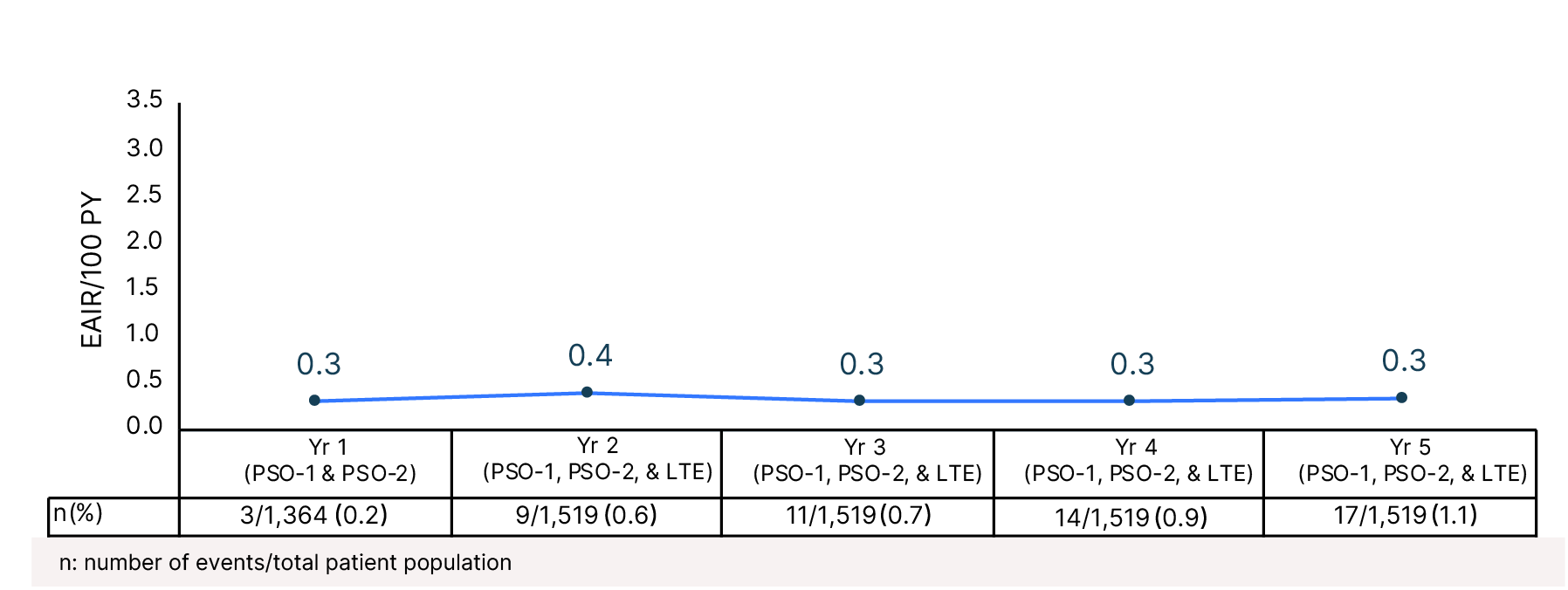

Cumulative Year-by-Year EAIRs for �MACE (as-treated population)29,31,35,37,38
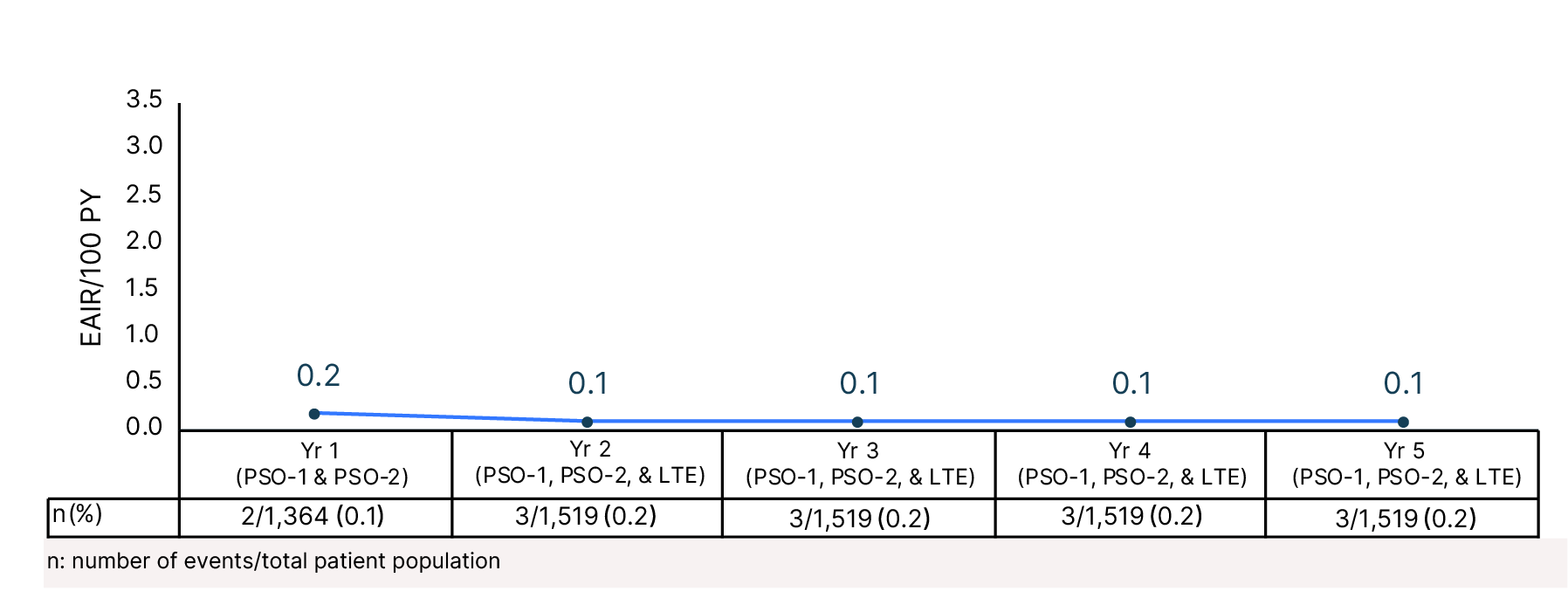

Cumulative Year-by-Year EAIRs for �VTE (as-treated population)29,31,35,37,38
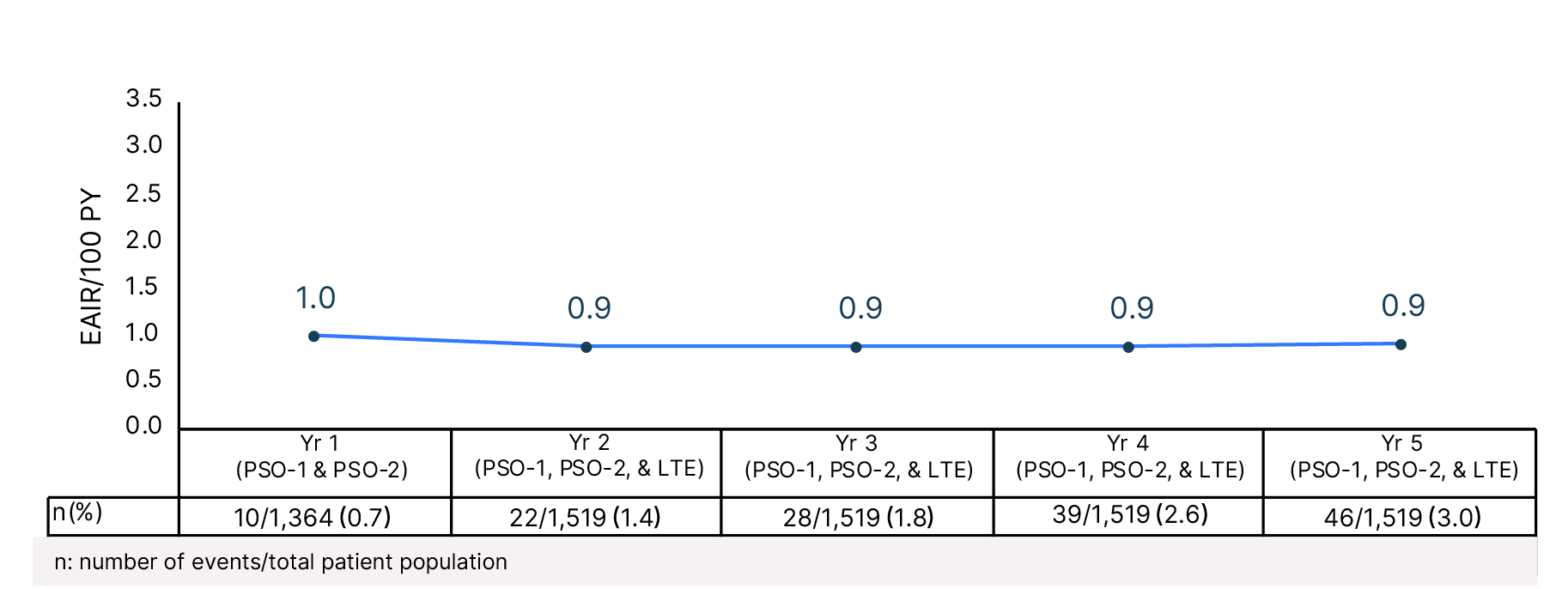

Cumulative Year-by-Year EAIRs for�Malignancies (as-treated population)29,31,35,37,38

PASI 75 for deucravacitinib vs apremilast: PSO-1: 69% (n=228/330) vs 38% (n=64/168), �P<0.0001; PSO-2: 58% (n=296/511) vs 38% (n=96/254), P<0.0001
PASI 90 for deucravacitinib vs apremilast: PSO-1: (n=140/330) vs 22% (n=37/168), �P<0.0001; PSO-2: 32% (n=164/511) vs 20% (n=50/254), P<0.0004
sPGA 0/1 for deucravacitinib vs apremilast: PSO-1: 59% (n=194/330) vs 31% (n=52/168), P<0.0001 vs apremilast32; PSO-2 49% (n=251/511) vs 30% (n=75/254), P<0.0001 vs apremilast39

PASI 75 response rates (mNRI) in the efficacy population:
• Start of LTE (52 weeks): 72.1% (95% CI: 68.2–76.1)
• At 256 weeks: 67.3% (95% CI: 62.0–72.6)
PASI 90 response rates (mNRI) in the efficacy population:
• Start of LTE (52 weeks): 45.9% (95% CI: 41.5–50.4)
• At 256 weeks: 46.3% (95% CI: 41.2–51.5)
sPGA 0/1 response rates (mNRI) in the efficacy population:
• Start of LTE (52 weeks): 57.5% (95% CI: 53.1–61.9)
• At 256 weeks: 52.6% (95% CI: 47.0–58.1)
mNRI: modified nonresponder imputation. For mNRI analyses, if there are no missing data (i.e., no imputed values in the dataset), 95% CI was obtained using the Clopper-Pearson method based on the observed data.
In POETYK PSO-LTE, deucravacitinib PASI response rates were maintained through 256 weeks.26
LTE LIMITATION: In open-label LTEs, patients who lose response or are unable to tolerate treatment are likely to discontinue treatment, which may raise the proportion of responders in the overall population. Data are derived from a post-hoc sub-analysis of POETYK PSO-LTE and include only patients from POETYK PSO-1 and PSO-2 who received continuous deucravacitinib from Day 1 (Week 0) and entered the LTE (n = 513, entered; n = 485, mNRI). Outcomes were analyzed descriptively. Note that the deucravacitinib arm of PSO-2 had forced re-randomization of half of the PASI 75 responders at Week 24; these patients are not included in this analysis, as they were no longer under continuous treatment.

Abbreviations
AE: adverse event; AESI: adverse event of special interest; ATP: adenosine triphosphate; BID: twice daily; CPK: creatine phosphokinase; EAIR: �exposure-adjusted incidence rate; EPO: erythropoietin; FDA: United States Food and Drug Administration; GH: growth hormone; GM-CSF: granulocyte macrophage-colony stimulating factor; HZ: herpes zoster; ICD: International Classification of Diseases; IFN: interferon; IL: interleukin; JAK: janus kinase; LTE: long-term extension; MACE: major adverse cardiovascular events; mDC: myeloid dendritic cell; mRNI: modified non-responder imputation; NMSC: non-melanoma skin cancer; PASI 75/90/100: ≥75%/90%/100% reduction from baseline in Psoriasis Area and Severity Index; PI: prescribing information; PsO: psoriasis; PY: �patient-years; QD: once daily; RA: rheumatoid arthritis; Rx: prescription; SAE: serious adverse event; sPGA 0/1: static Physician Global Assessment score of 0 (clear) or 1 (almost clear) with a ≥2-point improvement from baseline; STAT: signal transducer and activator of transcription; Th: helper T; TNF: tumor necrosis factor; TPO: thrombopoietin; TRx: total prescription; TYK2: tyrosine kinase 2; URTI: upper respiratory tract infection; vs: versus; VTE: venous thromboembolism; Yr: year.

1. Banerjee S et al. Drugs. 2017;77:521-46.
2. Wrobleski ST et al. J Med Chem. 2019;62:8973-95.
3. Tokarski JS et al. J Biol Chem. 2015;290:11061-74.
4. Ghoreschi K et al. Immunol Rev. 2009;228:273-87.
5. Clark JD et al. J Med Chem. 2014;57:5023-38.
6. Morris R et al. Protein Sci. 2018;27:1984-2009.
7. Dendrou CA et al. Sci Transl Med. 2016;8:363ra149.
8. Hammarén HM et al. Cytokine. 2019;118:48-63.
9. Krolopp JE et al. Front Physiol. 2016;7:626.
10. Xu D et al. JAKSTAT. 2013;2:e27203.
11. Richard AJ et al. Trends Endocrinol Metab. 2011;22:325-32.
12. Baker KF, Isaacs JD. Ann Rheum Dis. 2018;77:175-87.
13. Blauvelt A, Chiricozzi A. Clin Rev Allergy Immunol. 2018;55:379-90.
14. Chen K et al. J Autoimmun. 2017;83:1-11.
References

15. Gonciarz M et al. Immunotherapy. 2021;13:1135-50.�
16. Hawkes JE et al. J Allergy Clin Immunol. 2017;140:645-53.
17. Di Cesare A et al. J Invest Dermatol. 2009;129:1339-50.
18. Coskun M et al. Pharmacol Res. 2013;76:1-8.
19. Delgoffe GM, Vignali DAA. JAKSTAT. 2013;2:e23060.
20. Krueger JG et al. J Am Acad Dermatol. 2022;86:148-157.
21. Papp K et al. N Engl J Med. 2018;379:1313-21.
22. Borzilleri RM et al, Burger’s Medicinal Chemistry, Drug Discovery�and Development (2021) 8th edition. John Wiley & Sons.
23. Tokarski JS et al. J Biol Chem. 2015;290:11061-74.
24. Nogueira M et al. Drugs. 2020;80:341-52.
25. SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
26. Chimalakonda A et al. Dermatol Ther (Heidelb). 2021;11:1763-76.
27. Armstrong AW et al. J Am Acad Dermatol. 2023;88:29-39.

28. Strober B et al. J Am Acad Dermatol. 2023;88:40-51.
29. Armstrong AW et al. Oral presentation at EADV Spring Symposium 2024;�May 16-18, 2024; St. Julian’s, Malta.
30. Armstrong AW et al. Presentation at WCDC 2025; February 14-19, 2025;�Waikoloa, HI.
31. Data on file. BMS-REF-DEU-0049. Princeton, NJ: Bristol-Myers Squibb Company. 2022.
32. Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
33. Korman NJ et al. Poster presentation at the 31st EADV Congress; �September 7-10, 2022; Milan, Italy. Poster P1481.
34. Armstrong AW et al. Oral presentation at EADV; October 11-14 2023; Berlin, Germany. Abstract FC.02.7.
35. Strober B et al. J Eur Acad Dermatol Venereol. 2024;38:1543-54.

36. Lebwohl M et al. Br J Dermatol. 2024;190:668-79.
37. Data on file. BMS-REF-DEU-0127. Princeton, NJ: Bristol-Myers Squibb Company. 2024.
38. Data on file, BMS-REF-DEU-0164. Princeton, NJ: Bristol-Myers Squibb Company; 2023.
39. Data on file. BMS-REF-DEU-0021. Princeton, NJ: Bristol-Myers Squibb Company; 2022.


Deucravacitinib inhibits TYK2 implicated in �the pathogenesis of psoriasis
Extracellular
Deucravacitinib locks TYK2 in the inactive state inhibiting TYK2 �activation and its downstream activation of STATs15,20,22-24
(inactive state)
IL-17 production is decreased





The precise mechanism linking inhibition of TYK2 enzyme to therapeutic effectiveness in the treatment of adults with moderate-to-severe plaque psoriasis is not currently known.


Key Learnings
Deucravacitinib is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action, and is the first in a new class of small molecules.1


Deucravacitinib has been shown �to selectively target the�TYK2 protein kinase.26



Deucravacitinib is a once-daily �oral option for moderate-to-severe plaque psoriasis with 5 years of �data available.25,30



Study Design27-29,33,34
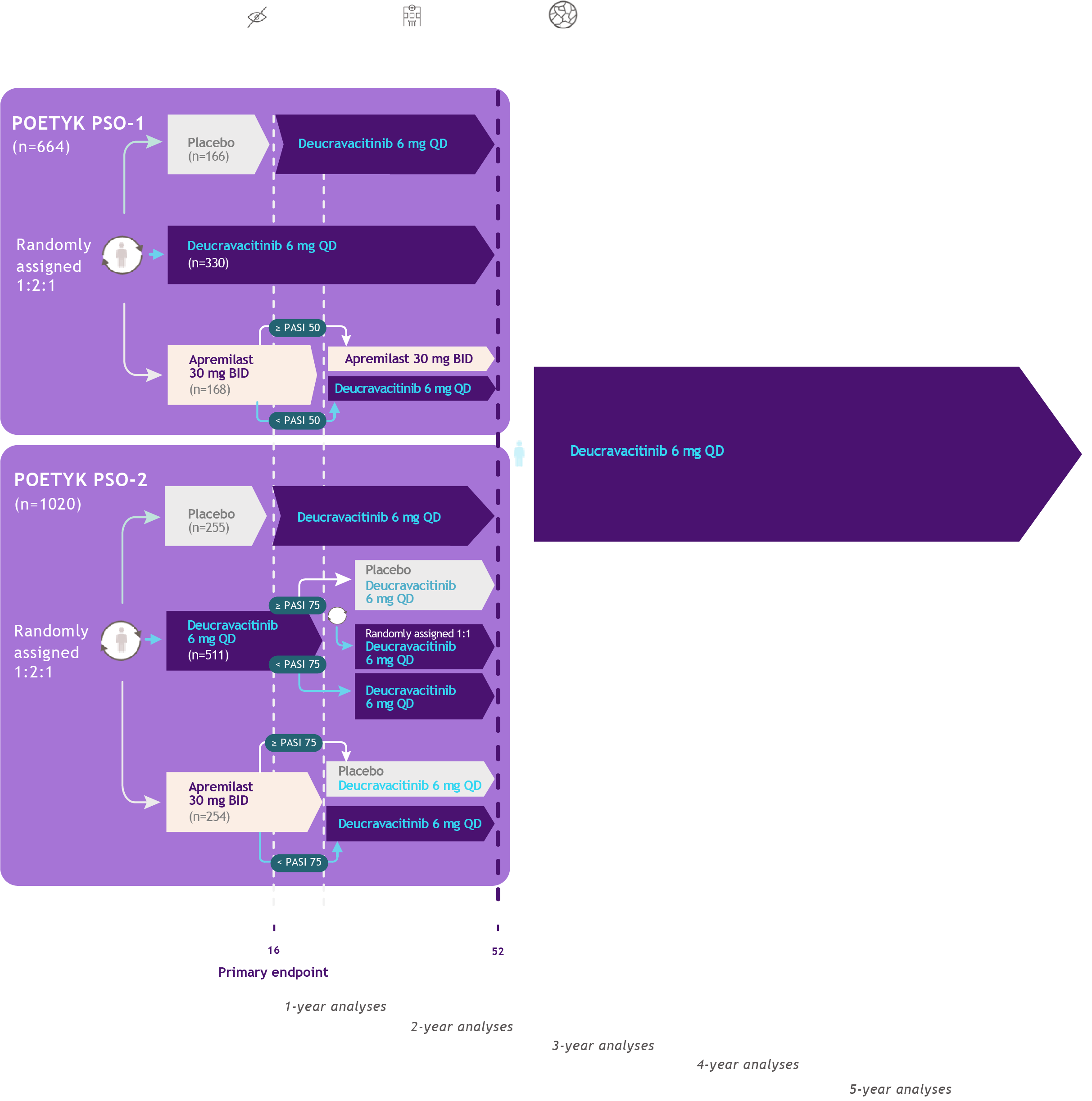
POETYK
PSO-1 & PSO-2
Co-primary endpoints�Proportion of patients who�achieve the following responses�versus placebo at Week 16:
sPGA score of 0 (clear)�or 1 (almost clear)
At least a 75% improvement�in PASI scores from�baseline (PASI 75)
Select secondary endpoints
Proportion of patients who achieve the �following responses:
At Week 24: PASI 75, PASI 90, sPGA 0/1
PASI 75: ≥75% reduction from baseline�in Psoriasis Area and Severity Index
PASI 90: >90% reduction from baseline in�Psoriasis Area and Severity Index
sPGA 0/1: static Physician Global Assessment �score of 0 (clear) or 1 (almost clear) with a �≥2-point improvement from baseline
POETYK PSO-LTE
Primary endpoint:
Incidence of AEs �and SAEs
Secondary endpoints:
PASI 75
PASI 90
POETYK
PSO-1 & PSO-2
Co-primary endpoints�Proportion of patients who�achieve the following responses�versus placebo at Week 16:
sPGA score of 0 (clear)�or 1 (almost clear)
At least a 75% improvement�in PASI scores from�baseline (PASI 75)
Select secondary endpoints
Proportion of patients who achieve the �following responses:
At Week 24: PASI 75, PASI 90, sPGA 0/1
PASI 75: ≥75% reduction from baseline�in Psoriasis Area and Severity Index
PASI 90: >90% reduction from baseline in�Psoriasis Area and Severity Index
sPGA 0/1: static Physician Global Assessment �score of 0 (clear) or 1 (almost clear) with a �≥2-point improvement from baseline
POETYK PSO-LTE
Primary endpoint:
Incidence of AEs �and SAEs
Secondary endpoints:
PASI 75
PASI 90

Study Design27-29,33,34

POETYK
PSO-1 & PSO-2
Co-primary endpoints�Proportion of patients who�achieve the following responses�versus placebo at Week 16:
sPGA score of 0 (clear)�or 1 (almost clear)
At least a 75% improvement�in PASI scores from�baseline (PASI 75)
Select secondary endpoints
Proportion of patients who achieve the �following responses:
At Week 24: PASI 75, PASI 90, sPGA 0/1
PASI 75: ≥75% reduction from baseline�in Psoriasis Area and Severity Index
PASI 90: >90% reduction from baseline in�Psoriasis Area and Severity Index
sPGA 0/1: static Physician Global Assessment �score of 0 (clear) or 1 (almost clear) with a �≥2-point improvement from baseline
POETYK PSO-LTE
Primary endpoint:
Incidence of AEs �and SAEs
Secondary endpoints:
PASI 75
PASI 90

Study Design27-29,33,34

POETYK
PSO-1 & PSO-2
Co-primary endpoints�Proportion of patients who�achieve the following responses�versus placebo at Week 16:
sPGA score of 0 (clear)�or 1 (almost clear)
At least a 75% improvement�in PASI scores from�baseline (PASI 75)
Select secondary endpoints
Proportion of patients who achieve the �following responses:
At Week 24: PASI 75, PASI 90, sPGA 0/1
PASI 75: ≥75% reduction from baseline�in Psoriasis Area and Severity Index
PASI 90: >90% reduction from baseline in�Psoriasis Area and Severity Index
sPGA 0/1: static Physician Global Assessment �score of 0 (clear) or 1 (almost clear) with a �≥2-point improvement from baseline
POETYK PSO-LTE
Primary endpoint:
Incidence of AEs �and SAEs
Secondary endpoints:
PASI 75
PASI 90

Study Design27-29,33,34

Important Safety Information

CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
Important Safety Information

CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Infections: SOTYKTU may increase the risk of infections. Serious infections have been reported in patients with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19. Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
with chronic or recurrent infection
who have been exposed to tuberculosis
with a history of a serious or an opportunistic infection
with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment. �A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing, have appropriate antimicrobial therapy initiated and be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.

Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex) was reported in clinical trials with SOTYKTU. Through Week 16, herpes simplex infections were reported in 17 patients (6.8 per 100 patient-years) treated with SOTYKTU, and 1 patient (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent patient. During PSO-1, PSO-2, and the open-label extension trial, the majority of patients who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age. The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis (TB): In clinical trials, of 4 patients with latent TB who were treated with SOTYKTU and received appropriate TB prophylaxis, no patients developed active TB (during the mean follow-up of 34 weeks). One patient, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU. Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas: Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.


Rhabdomyolysis and Elevated CPK: Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Laboratory Abnormalities: Treatment with SOTYKTU was associated with increases in triglyceride levels. Periodically evaluate serum triglycerides according to clinical guidelines during treatment. SOTYKTU treatment was associated with an increase in the incidence of liver enzyme elevation compared to placebo. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded.
Immunizations: Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.


ADVERSE REACTIONS
Most common adverse reactions (≥1% of patients on SOTYKTU and more frequently than with placebo) include upper respiratory infections, blood creatine phosphokinase increased, herpes simplex, mouth ulcers, folliculitis and acne.
SPECIFIC POPULATIONS
Pregnancy: Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Lactation: There are no data on the presence of SOTYKTU in human milk, the effects on the breastfed infant, or the effects on milk production. SOTYKTU is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Hepatic Impairment: SOTYKTU is not recommended for use in patients with severe hepatic impairment.
SOTYKTU is available in 6 mg tablets.


INDICATION
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
Please see U.S. Full Prescribing Information, including Medication Guide, for SOTYKTU.













IMM-US-2400448 May 2025