IMFINZI plus carboplatin and paclitaxel (CP) brings another treatment option to patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient


SCROLL TO CONTINUE


As of June 2024, the FDA approved IMFINZI® (durvalumab) in combination with carboplatin and paclitaxel (CP) followed by IMFINZI as a single agent for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR).

Exploring the DUO-E Indication and Clinical Data With Principal Investigator Dr Shannon Westin


WATCH NOW
IMFINZI + CP followed by IMFINZI as a single agent led to a


58% reduction
in the risk of progression or death in patients �with dMMR endometrial cancer �(HR=0.42; 95% CI, 0.22-0.80)

Median PFS was NR�with IMFINZI + CP�(95% CI, NR-NR)
Median PFS was 7.0 months�with CP�(95% CI, 6.7-14.8)
FDA approval was based on a prespecified dMMR subgroup (n=95). The prespecified PFS subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups.
CI=confidence interval; CP=carboplatin and paclitaxel; dMMR=mismatch repair deficient; FDA=United States Food and Drug Administration; HR=hazard ratio; NR=not reached; PFS=progression-free survival.
CLICK TO EXPAND

Unmet Need and Recent Advancements


SCROLL TO CONTINUE


Endometrial cancer (EC) is the most common gynecologic malignancy in the US, with rising incidence and mortality

There is an unmet need for more treatment options


The endometrial cancer treatment landscape is shifting


Mismatch repair status is an established prognostic biomarker



In the US:
EC is the 4th most common cancer in women
EC accounts for 6% of cancers in women
Approximately 69,120 new cases of EC will be diagnosed in 2025
Approximately 13,860 women will die from EC in 2025
The number of new cases will double to 122,000 cases per year by 2030 if current trends continue
The rising incidence of endometrial cancer may be associated with several risk factors, including obesity, diabetes, and changes in hormone therapy use.


Endometrial cancer (EC) is the most common gynecologic malignancy in the US, with rising incidence and mortality

Patients diagnosed with localized disease have a 5-year survival rate of approximately 95%, whereas the survival rate falls below 20% for those with metastatic or recurrent disease.
Considering the rising incidence and mortality, it is more important than ever to bring �additional treatment options to patients with endometrial cancer.

There is an unmet need for more treatment options

Approximately 18% of women with endometrial cancer experience disease recurrence.
There is a need for additional treatment options for patients with primary advanced or recurrent dMMR endometrial cancer.

Historically, standard of care for advanced or recurrent endometrial cancer has been platinum-based chemotherapy with carboplatin plus paclitaxel.
However, outcomes for patients with advanced or recurrent endometrial cancer remain poor when treated �with conventional chemotherapy regardless of mismatch repair (MMR) status, with 5-year survival rates �below 20%.

The endometrial cancer treatment landscape is shifting

Testing patients for mismatch repair (MMR) status is recommended soon after diagnosis, as MMR status dictates if patients are eligible to receive specific targeted therapies.
Patients can be MMR deficient (dMMR) or proficient (pMMR) in the MMR pathway that repairs DNA damage. �The MMR pathway corrects base mismatches and insertion or deletion mismatches that can occur during DNA replication, and therefore plays an important role in maintaining genomic stability. Defects in MMR increases mutation rates and can be oncogenic. dMMR tumors are more likely to produce abnormal proteins that attract immune cells and are generally more responsive to immunotherapy treatment, in comparison to pMMR tumors.

Mismatch repair status is an established prognostic biomarker


Exploring the DUO-E Indication and Clinical Data With Principal Investigator Dr Shannon Westin
Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center
Dr Westin describes the DUO-E efficacy and safety data that supported the FDA approval in primary advanced or recurrent dMMR endometrial cancer. Furthermore, Dr Westin shares insights into how physicians can translate these findings into another treatment option for patients with primary advanced or recurrent dMMR endometrial cancer.


WATCH NOW



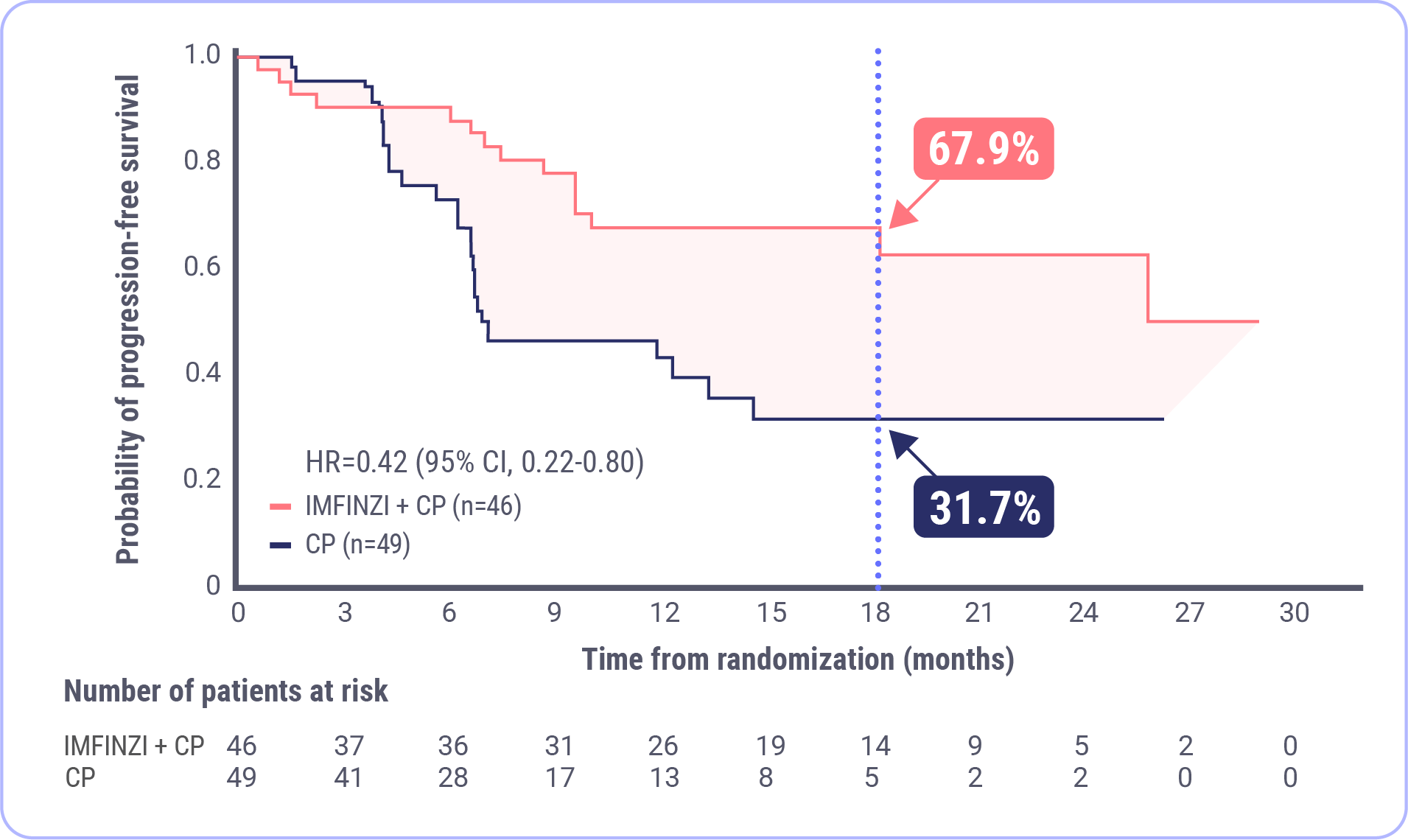
PFS in the dMMR Subgroup
IMFINZI in combination with carboplatin and paclitaxel (CP) followed by IMFINZI monotherapy led to a clinically meaningful reduction in the risk of disease progression or death by 58% (HR=0.42; 95% CI, 0.22-0.80).
Prespecified Exploratory Analysis of PFS in the dMMR Subgroup

CLICK TO ZOOM
58% Reduction in the Risk of Progression or Death�with IMFINZI + CP �(HR=0.42 [95% CI, 0.22-0.80])
NR mPFS�with IMFINZI + CP�(95% CI, NR-NR)
7.0 month mPFS�with CP�(95% CI, 6.7-14.8)
and
At 18 months, 67.9% of patients were estimated to be alive and progression free in the IMFINZI + CP arm and 31.7% were estimated to be alive and progression free in the CP arm.
FDA approval was based on a prespecified dMMR subgroup (n=95). The prespecified PFS subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups.
Median duration of follow-up: 15.5 months for IMFINZI + CP; 10.2 months for CP.�Data cutoff: April 12, 2023.
CI=confidence interval; CP=carboplatin and paclitaxel; dMMR=mismatch repair deficient; FDA=United States Food and Drug Administration; HR=hazard ratio; mPFS=median progression-free survival; NR=not reached; �PFS=progression-free survival.
Swipe for more clinical data


X


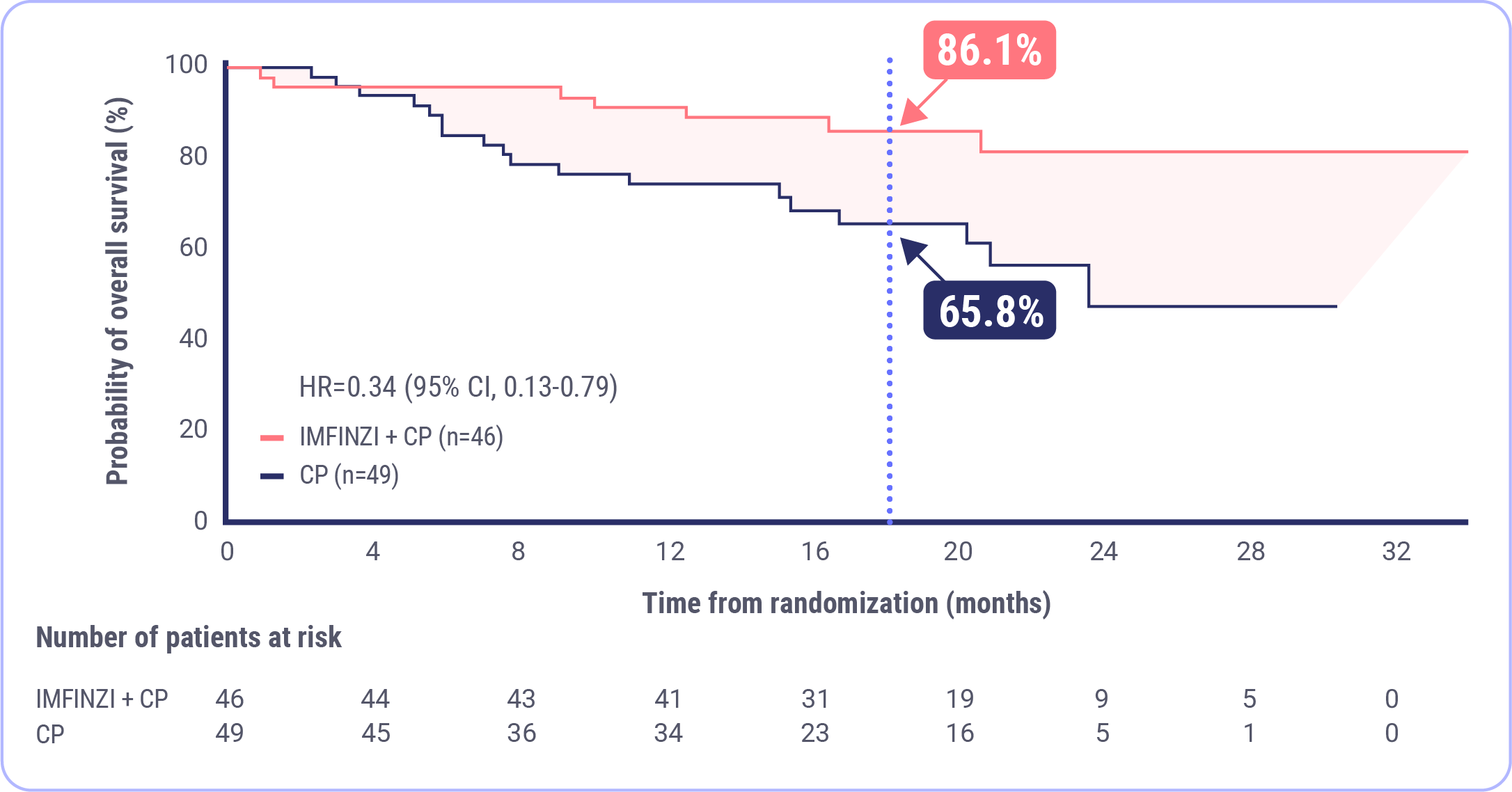
OS Results at Interim Analysis in the dMMR Subgroup
Post-hoc Exploratory Analysis of OS in dMMR Subgroup

CLICK TO ZOOM
NR mOS�with IMFINZI + CP�(95% CI, NR-NR)
23.7 month mOS�with CP�(95% CI, 16.9-NR)
and
At 18 months, 86.1% of patients were estimated to be alive in the IMFINZI + CP arm and 65.8% were estimated to be alive in the CP arm.
FDA approval was based on a prespecified dMMR subgroup (n=95). The post-hoc OS subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups.
OS was immature at 26%�Median duration of follow-up: 19.1 months for IMFINZI + CP and 18.4 months for CP.�Data cutoff: April 12, 2023.
CI=confidence interval; CP=carboplatin and paclitaxel; dMMR=mismatch repair deficient; FDA=United States Food and Drug Administration; HR=hazard ratio; mOS=median overall survival; NR=not reached; OS=overall survival.

X


ORR in the dMMR Subgroup
The objective response rate (ORR) was 71.4% in the IMFINZI + CP arm and 40.5% in the CP arm.
Post-hoc Exploratory Analysis of ORR in dMMR Subgroup
FDA approval was based on a prespecified dMMR subgroup (n=95). The post-hoc ORR subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups.
Data cutoff: April 12, 2023
CI=confidence interval; CP=carboplatin and paclitaxel; CR=complete response; dMMR=mismatch repair deficient; FDA=United States Food and Drug Administration; ORR=objective response rate; PR=partial response.



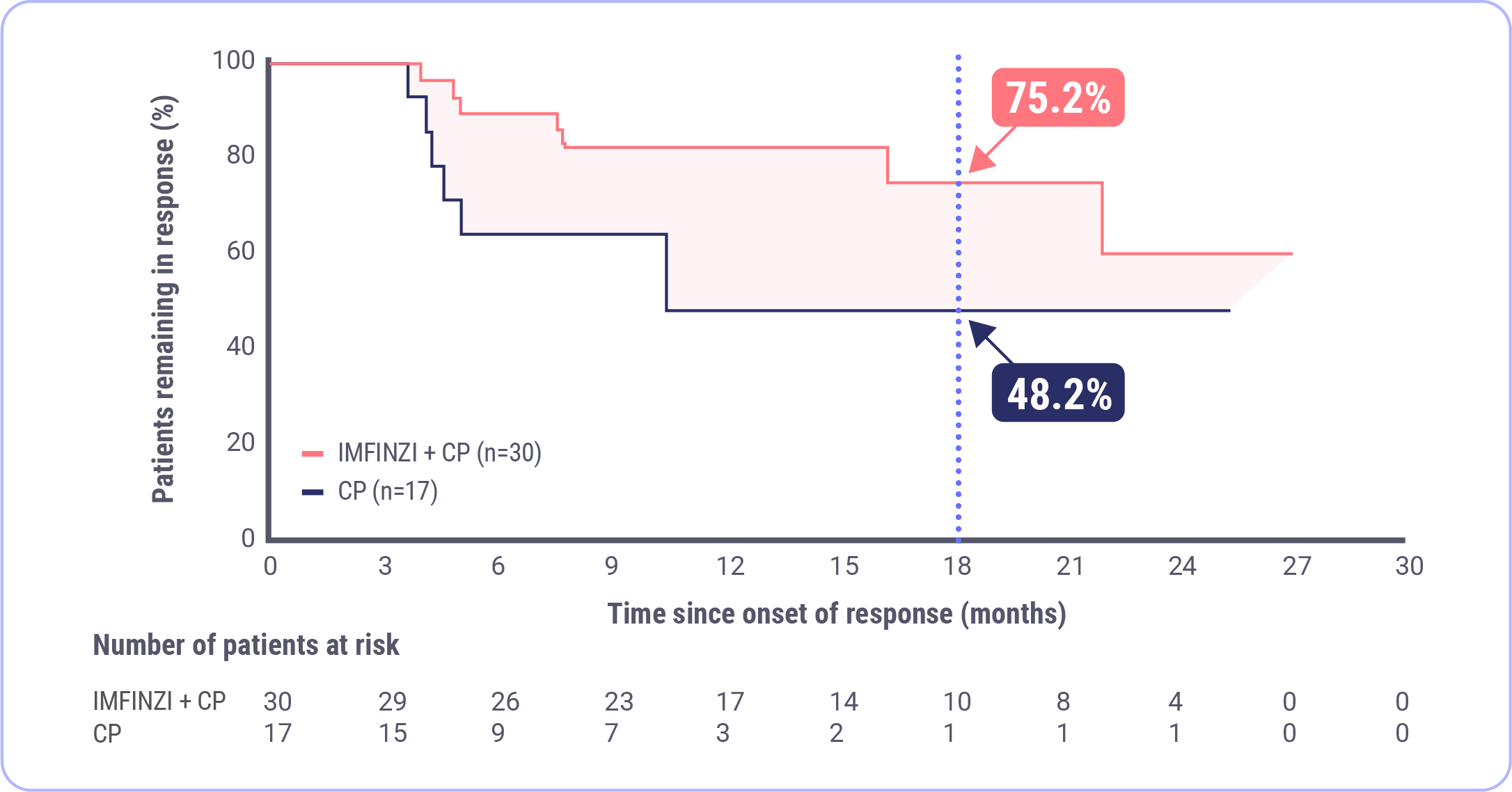
DoR in the dMMR Subgroup
Post-hoc Exploratory Analysis of DoR in dMMR Subgroup

CLICK TO ZOOM
NR mDoR�with IMFINZI + CP�(range: 2.4+, 26.9+)
10.5 month mDoR�with CP�(range: 2.1+, 25.2+)
and
FDA approval was based on a prespecified dMMR subgroup (n=95). The post-hoc DoR subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups.
Data cutoff: April 12, 2023
CI=confidence interval; CP=carboplatin and paclitaxel; dMMR=mismatch repair deficient; DoR=duration of response; FDA=United States Food and Drug Administration; HR=hazard ratio; mDoR=median duration of response; NR=not reached.
The median duration of response (DoR) was not reached in the IMFINZI + CP arm and 10.5 months in the CP arm.

X




WATCH NOW
The DUO-E Study Design: Physician Perspectives on Key Features
Dr Shannon Westin �Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center
Dr Floor Backes�Professor, Division of Gynecologic Oncology and Director of Gynecologic Cancer Research at The Ohio State University
Dr Westin and Dr Backes explore the details of key DUO-E study design features and offer expert opinions on how these features may influence trial results, interpretations, and implementation into clinical practice.

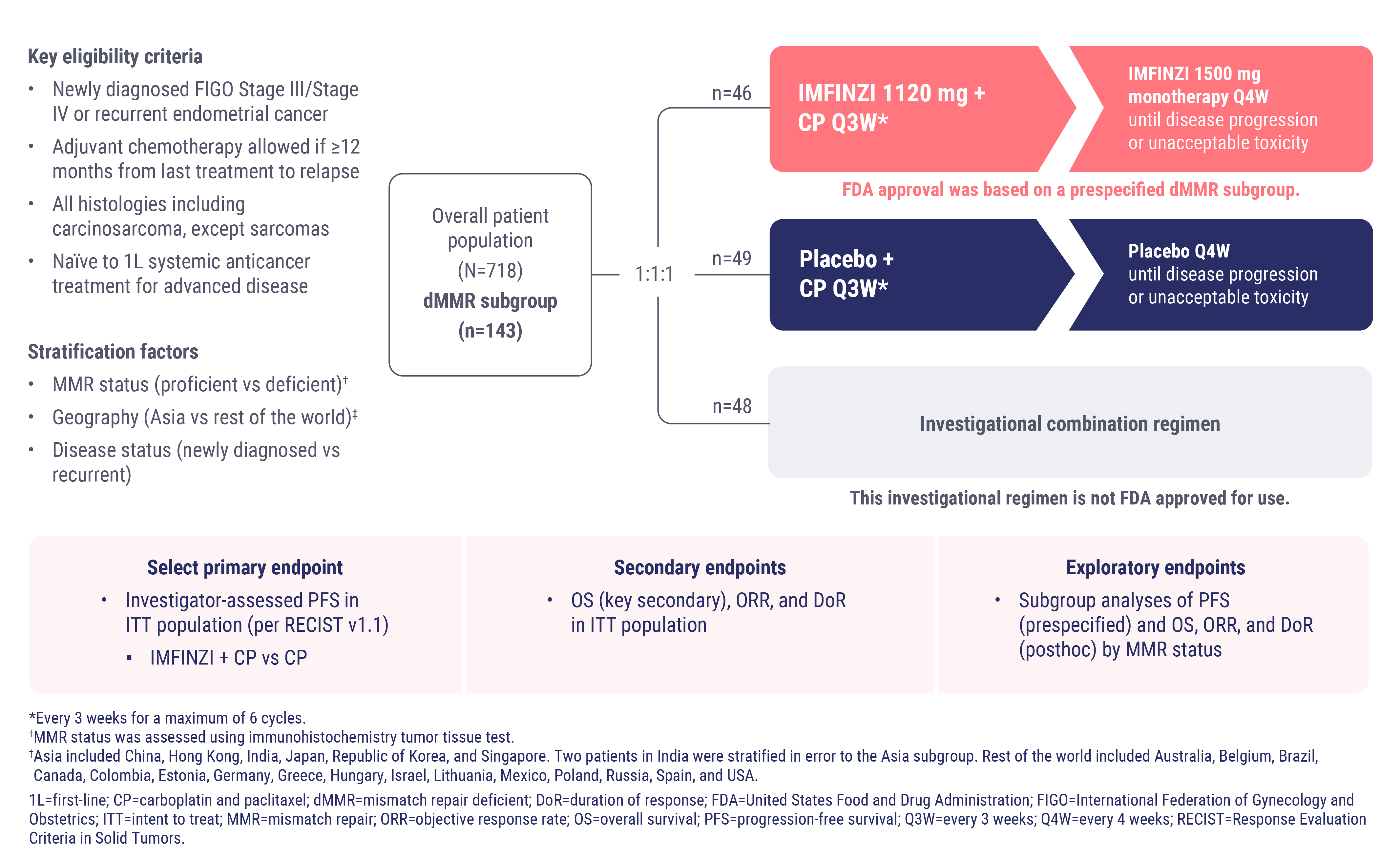
DUO-E Trial Design
DUO-E was a global, randomized, double-blind, placebo-controlled Phase III study

CLICK TO ZOOM

X



WATCH NOW
Exploring Adverse Events in DUO-E
Dr Shannon Westin �Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center
Dr Floor Backes�Professor, Division of Gynecologic Oncology and Director of Gynecologic Cancer Research at The Ohio State University
Dr Westin and Dr Backes review details of the DUO-E safety data and offer expert perspectives on how to monitor and help manage select immune-mediated adverse events.


Safety in the dMMR Subgroup
Safety data for IMFINZI + CP are available for a total of 44 patients with advanced or recurrent dMMR endometrial cancer.
Patients received IMFINZI (1120 mg) with CP (every 3 weeks for up to six 21-day cycles) followed by IMFINZI (1500 mg every 4 weeks [or CP every 3 weeks for up to six 21-day cycles alone]). Treatment was continued until disease progression or unacceptable toxicity.
The median duration of exposure to IMFINZI with CP was 14.8 months (range: 0.7 to 31.7).�



Serious adverse reactions (ARs) occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel�
The most common serious ARs defined as those occurring in 4% of patients or more were constipation and rash, which both occurred in 4.5% of patients
Permanent discontinuation of IMFINZI due to ARs occurred in 11% of patients�
The AR that resulted in permanent discontinuation of IMFINZI in 4% or more of patients was rash �at 4.5%
Dosage interruptions of IMFINZI due to ARs occurred in 52% of patients
ARs that required dosage interruptions of IMFINZI in 4% or more of patients were:
Anemia (11%)
Thrombocytopenia (9%)
Neutropenia (9%)
COVID-19 (9%)
Increased alanine transaminase (ALT) (4.5%)
Pneumonitis (4.5%)
Clinically relevant ARs in less than 10% of patients who received IMFINZI with carboplatin and paclitaxel included:
Autoimmune hemolytic anemia
Colitis
Immune-mediated thyroiditis
Infusion-related reaction
Interstitial lung disease
Myositis
Pneumonitis
Pulmonary embolism
Sepsis
Please see the IMFINZI Prescribing Information for further details.
IMFINZI + CP offers another option to treat patients �with primary advanced or recurrent dMMR EC

IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient as determined by an FDA-approved test.
DUO-E Efficacy
The FDA approval of IMFINZI is supported by clinically meaningful data from DUO-E, which demonstrated that the combination of IMFINZI and CP reduced the risk of progression or death by more than half in patients with dMMR endometrial cancer (n=95). The prespecified PFS subgroup analysis was exploratory and not designed to assess a statistical difference between treatment groups
IMFINZI + CP led to a 58% reduction in the risk of progression or death, with a hazard ratio of 0.42 (95% CI, 0.22-0.80)
The median progression-free survival was not reached in the IMFINZI + CP arm (95% CI, NR-NR) and was 7 months in the CP arm (95% CI, 6.7-14.8)
Testing patients to determine mismatch repair status at diagnosis is recommended to evaluate if patients are eligible to receive precision medicine treatments
Select patients for treatment based on the presence of dMMR in tumor specimens. Information on FDA-approved tests for the detection of dMMR status in endometrial cancer is available at https://www.fda.gov/companiondiagnostics.
DUO-E Study Design
In the DUO-E study, there are several trial design features that may influence results and interpretations. These features include:
Geographic location diversity
Inclusion of characteristics associated with aggressive disease
The platinum-free interval for patients with recurrent disease
Treatment duration
The statistical analysis plan
DUO-E Safety
Serious ARs occurred in 30% of patients who received IMFINZI + CP. The most common serious ARs (≥4%) were constipation (4.5%) and rash (4.5%)
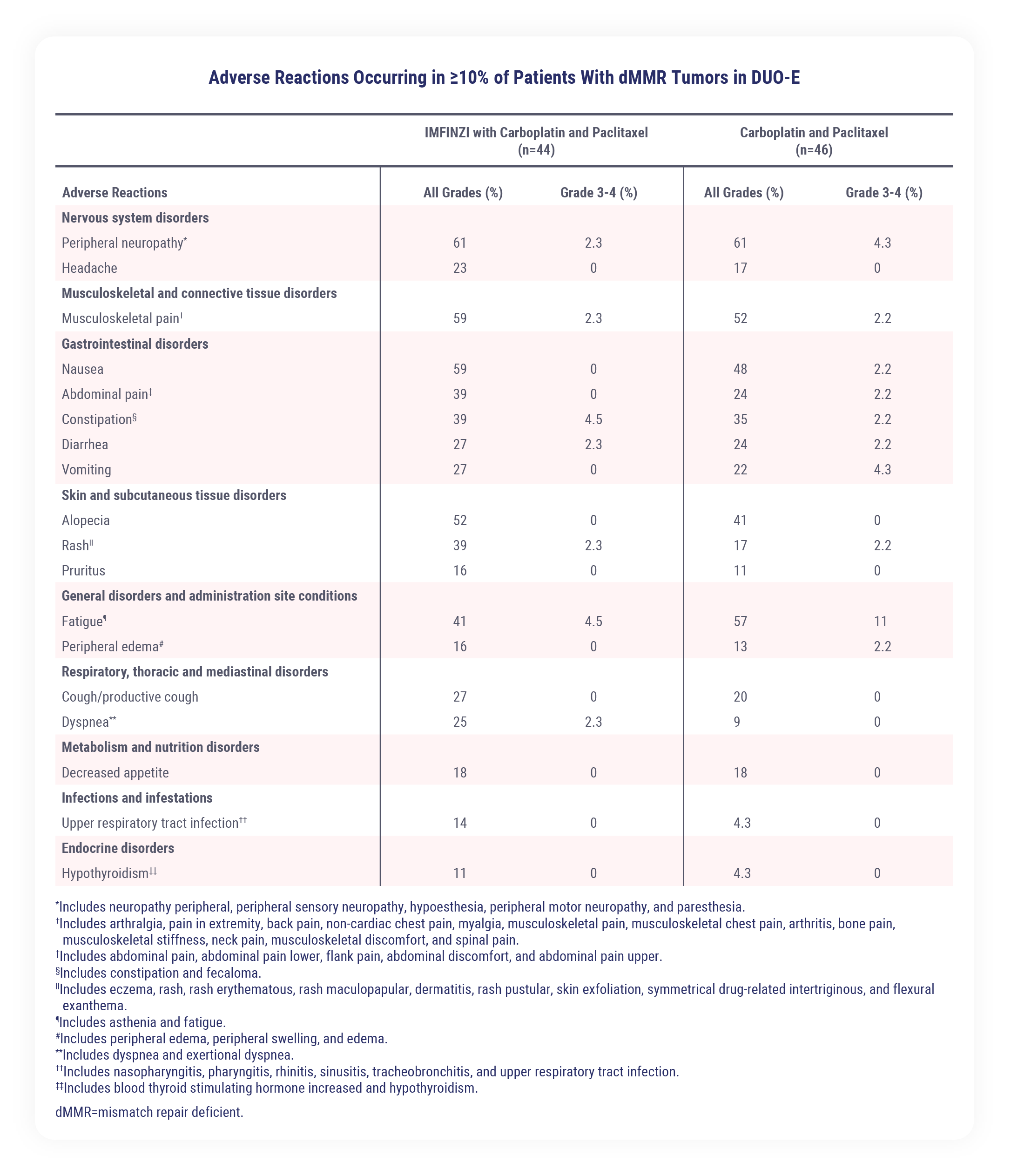
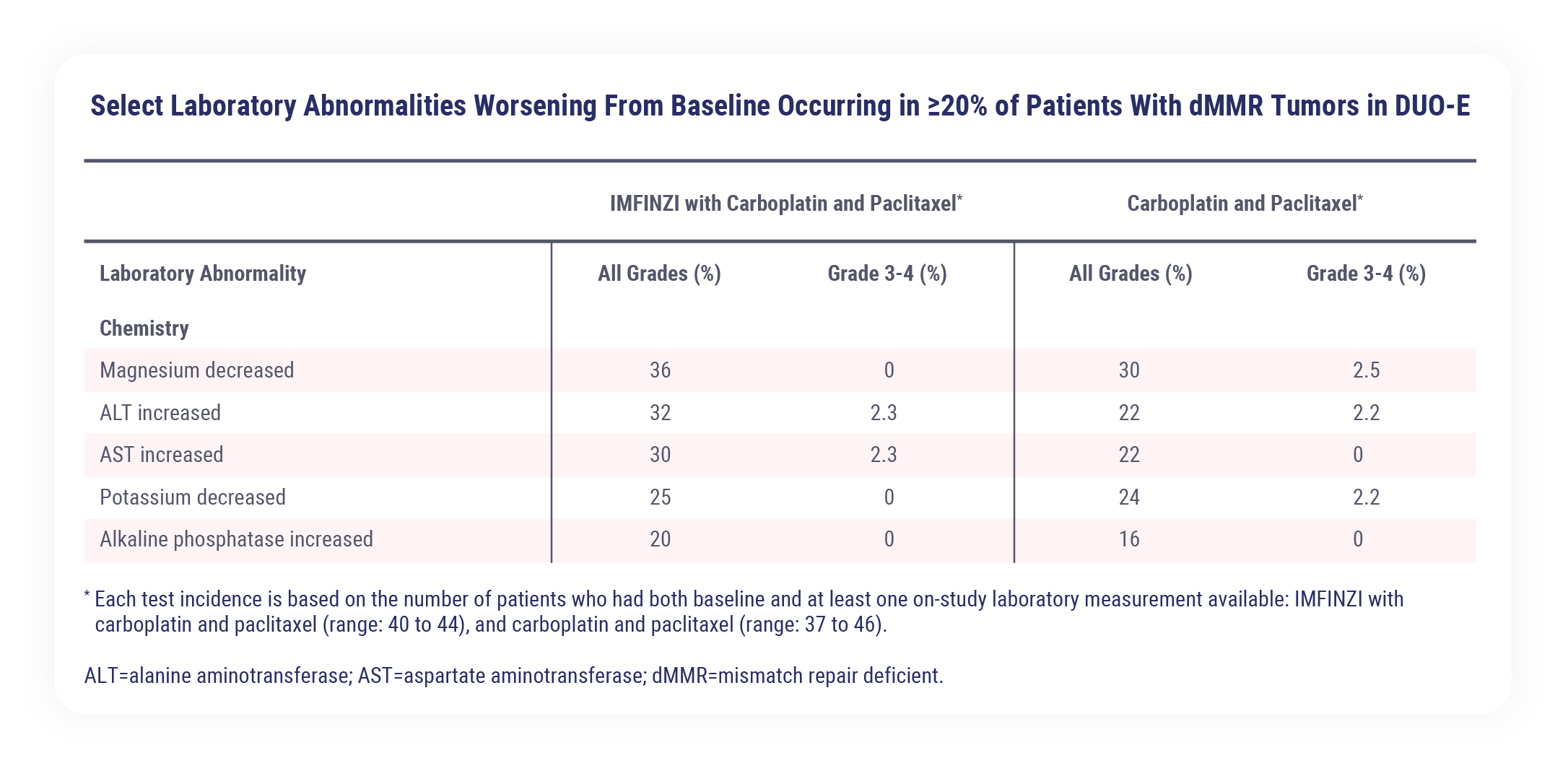
The most common adverse reactions (>20%), including laboratory abnormalities, were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, fatigue, abdominal pain, constipation, rash, decreased magnesium, increased ALT, increased AST, diarrhea, vomiting, cough, decreased potassium, dyspnea, headache, increased alkaline phosphatase, and decreased appetite
Permanent discontinuation of IMFINZI due to ARs occurred in 11% of patients. The AR that resulted in permanent discontinuation of IMFINZI in 4% or more of patients was rash at 4.5%
Dosage interruptions of IMFINZI due to ARs occurred in 52% of patients. ARs that required dosage interruptions of IMFINZI (≥4%) were anemia (11%), thrombocytopenia (9%), neutropenia (9%), COVID-19 (9%), increased ALT (4.5%), and pneumonitis (4.5%)
IMFINZI plus CP in EC
The IMFINZI plus CP combination indication offers an additional treatment option for patients with primary advanced or recurrent dMMR endometrial cancer, thus expanding the treatment options.
References

1L=first-line; ALT=alanine aminotransferase; ARs=adverse reactions; AST=aspartate aminotransferase; CI=confidence interval; CP=carboplatin and paclitaxel; dMMR=mismatch repair deficient; DoR=duration of response; EC=endometrial cancer; FDA=United States Food and Drug Administration; FIGO=International Federation of Gynecology and Obstetrics; HR=hazard ratio; ITT=intent to treat; MMR=mismatch repair; mOS=median overall survival; mPFS=median progression-free survival; NR=not reached; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; Q3W=every 3 weeks; Q4W=every 4 weeks; RECIST=Response Evaluation Criteria in Solid Tumors.

HCP Site
US Prescribing Information
Medical Information
AstraZeneca Privacy Notice

IMFINZI is a registered trademark of the AstraZeneca group of companies.�For US Healthcare Providers only. �©2025 AstraZeneca. All rights reserved. US-92561 Last Updated 8/25
Back to Top



IMFINZI Prescribing Information
MENU

MENU

jump to any topic

IMFINZI + CP in Endometrial Cancer


Unmet Need and Recent Advancements

Efficacy Video


DUO-E

Study Design Video


Safety and AE Management Video


Summary


Important Safety Information


Important Safety Information

Important Safety Information

There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune- ...


DUO-E Efficacy Video


Abbreviations


American Cancer Society (ACS). Cancer Facts & Figures. 2025. Accessed June 2025. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf
American Cancer Society (ACS). About Endometrial Cancer. Accessed June 2025. https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statistics.html
AstraZeneca. IMFINZI plus chemotherapy approved in the US for mismatch repair deficient advanced or recurrent endometrial cancer. Accessed June 2025. https://www.astrazeneca.com/media-centre/press-releases/2024/imfinzi-approved-in-the-us-for-endometrial-cancer.html
Baurain J-F, Chon HS, Thomes-Pepin J, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab +/- olaparib as a first-line treatment for endometrial cancer: overall survival and additional secondary efficacy endpoints by mismatch repair status in the DUO-E/GOG-3041/ENGOT-EN10 trial. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
Berg HF, Engerud H, Myrvold M, et al. Mismatch repair markers in preoperative and operative endometrial cancer samples; expression concordance and prognostic value. Br J Cancer. 2023;128(4):647-655.
Cao SY, Fan Y, Zhang YF, Ruan JY, Mu Y, Li JK. Recurrence and survival of patients with stage III endometrial cancer after radical surgery followed by adjuvant chemo- or chemoradiotherapy: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):31.
Chon HS, Thomes-Pepin J, Sundborg MJ, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab with or without olaparib as first-line treatment for endometrial cancer (DUO-E/GOG-3041/ENGOT-EN10): objective response rate and duration of response by mismatch repair status. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women's Health Initiative: An assessment of risk factors. J Womens Health (Larchmt). 2019;28(2):237-243.
Data on File, REF-232005, AstraZeneca Pharmaceuticals LP; 2024.
Data on File, REF-233132, AstraZeneca Pharmaceuticals LP; 2024.
Hamoud BH, Sima RM, Vacaroiu IA, et al. The evolving landscape of immunotherapy in uterine cancer: A comprehensive review. Life (Basel). 2023;13(7):1502.
IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130-162.
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18(1):85-98.
Li K, Luo H, Huang L, Luo H, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020;20:16.
Makker V, Green AK, Wenham RM, Mutch D, Davidson B, Miller DS. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4:19.
Makker V, MacKay H, Ray-Coquard I, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7(1):88.
Monk BJ, Smith G, Lima J, et al. Real-world outcomes in patients with advanced endometrial cancer: A retrospective cohort study of US electronic health records. Gynecol Oncol. 2022;164(2):325-332.
National Cancer Institute (NCI). Cancer Stat Facts: Uterine Cancer. Accessed June 2025. https://seer.cancer.gov/statfacts/html/corp.html
Siegenthaler F, Lindemann K, Epstein E, et al. Time to first recurrence, pattern of recurrence, and survival after recurrence in endometrial cancer according to the molecular classification. Gynecol Oncol. 2022;165(2):230-238.
Tillmanns T, Masri A, Stewart C, et al. Advanced endometrial cancer-The next generation of treatment: A society of gynecologic oncology journal club clinical commentary. Gynecol Oncol Rep. 2024;55:101462.
Westin SN, Moore K, Chon HS, et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283-299.
Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54.

There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal �immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated.
Thyroid Disorders (Thyroiditis, Hyperthyroidism, and Hypothyroidism): IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.


Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.


Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.


Adverse Reactions
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), the most common adverse reactions, including laboratory abnormalities (occurring in ≥20% of patients) were peripheral neuropathy (61%), musculoskeletal pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%). The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
Please see Full Prescribing Information including Medication Guide for IMFINZI.
You may report side effects related to AstraZeneca products .

This content is sponsored by AstraZeneca.
This content is intended only for US Healthcare Providers.
IMFINZI + CP in Primary Advanced or Recurrent dMMR Endometrial Cancer
DUO-E Summary
DUO-E Study Design Video
DUO-E Safety and AE Management Video

Exploring the DUO-E Indication and Clinical Data �With Principal Investigator Dr Shannon Westin
Dr Westin describes the DUO-E efficacy and safety data that supported the FDA approval in primary advanced or recurrent dMMR endometrial cancer. Furthermore, Dr Westin shares insights into how physicians can translate these findings into another treatment option for patients with primary advanced or recurrent dMMR endometrial cancer.


Speaker Bio:
Dr Shannon Westin
Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center

X
Exploring the DUO-E Indication and Clinical Data �With Principal Investigator Dr Shannon Westin
Dr Westin describes the DUO-E efficacy and safety data that supported the FDA approval in primary advanced or recurrent dMMR endometrial cancer. Furthermore, Dr Westin shares insights into how physicians can translate these findings into another treatment option for patients with primary advanced or recurrent dMMR endometrial cancer.


Speaker Bio:
Dr Shannon Westin
Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center

X
The DUO-E Study Design: �Physician Perspectives on Key Features
Dr Westin and Dr Backes explore the details of key DUO-E study design features and offer expert opinions on how these features may influence implementation into clinical practice.


Speaker Bios:
Dr Shannon Westin
Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center

X

Dr Floor Backes
Associate Professor, Division of Gynecologic Oncology at The Ohio State University
Exploring Adverse Events�in DUO-E
Dr Westin and Dr Backes review details of the DUO-E safety data and offer expert perspectives on how �to monitor and help manage select �adverse events.


Speaker Bios:
Dr Shannon Westin
Professor of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center
Dr Floor Backes
Associate Professor, Division of Gynecologic Oncology at The Ohio State University

X


ISI �continued
ISI �continued
ISI �continued
ISI �continued

Transcript
Introduction
Hello! Thank you for joining me today. I’m Dr Shannon Westin, Professor and Clinical Medical Director in the Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center.
And today we will discuss the US indication for IMFINZI in combination with carboplatin and paclitaxel in primary advanced or recurrent dMMR endometrial cancer and the data from the DUO-E trial that supported the FDA approval.
Before we proceed, I need to note that the opinions expressed in this video are solely my own and do not reflect those of AstraZeneca.
IMFINZI Endometrial Cancer Indication and Clinical Data
In June 2024, AstraZeneca received FDA approval for IMFINZI in combination with carboplatin and paclitaxel, known as CP, followed by IMFINZI as a single agent for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient, also known as dMMR.1,2
This approval was based on the prespecified exploratory analysis of progression-free survival in the dMMR subgroup that included 95 patients from the DUO-E trial. IMFINZI plus CP led to a 58% reduction in the risk of progression or death, with a hazard ratio of 0.42. The median progression-free survival was not reached in the IMFINZI plus CP arm and was 7 months in the CP arm.1,3
The IMFINZI-based regimen offers an additional treatment option to address a significant need for patients with advanced or recurrent endometrial cancer who have a five-year survival rate below 20%.1,4,5
The DUO-E data add to the body of evidence that support the use of immunotherapy plus CP for patients with endometrial cancer that is mismatch repair deficient. This underscores the importance of determining the mismatch repair status of the patient at diagnosis to offer a precision medicine approach to their treatment.1,4,6

X
DUO-E Study Design
The DUO-E study was a global, randomized, double-blind, placebo-controlled, Phase III study of 718 patients with advanced or recurrent endometrial cancer.3
Patients with recurrent disease were required to have at least a 12-month chemotherapy-free interval to the date of their subsequent relapse with chemotherapy being delivered in the adjuvant setting. All histologies were eligible including carcinosarcomas, except sarcomas.3
Within the dMMR subgroup, 95 patients received first-line treatment with CP plus IMFINZI (or placebo) at 1120 mg once every three weeks for 6 cycles, followed by maintenance IMFINZI (or placebo) at 1500 mg once every four weeks. All treatments were given until disease progression or unacceptable toxicity.3
For more details on the DUO-E trial design and how certain aspects may impact interpretations, consider watching the video called “The DUO-E Study Design: Physician Perspectives on Key Features.”
Patient Characteristics in the dMMR Subgroup
As for the patient baseline characteristics, these were generally balanced across treatment arms and representative of patients with newly diagnosed advanced or recurrent endometrial cancer.3
The DUO-E study was designed to enroll a patient group that is representative of the global real-world population. It included trial sites in North America, Europe and Asia, and included patients with advanced and/or aggressive disease.3,7
In the IMFINZI arm within the dMMR subgroup, 30% of patients enrolled were from Asia, while 70% of patients were from other areas around the world, labelled as non-Asia. This highlights the environmental and genetic diversity of the patients enrolled within the DUO-E study.3,8

X

Patients in both arms had characteristics associated with aggressive disease7:
37% had newly diagnosed Stage IV endometrial cancer
54% of patients had recurrent endometrial cancer
89% of patients had measurable disease at baseline, and
22% of patients had serous carcinoma, carcinosarcoma, mixed carcinoma, clear cell adenocarcinoma, or other histologies
Prespecified Exploratory Analysis of PFS in the dMMR Subgroup
As noted previously, FDA approval was based on the prespecified dMMR subgroup analysis of the DUO-E Phase III trial.1,3
In the dMMR subgroup, IMFINZI in combination with CP followed by IMFINZI as a single agent reduced the risk of disease progression or death by 58%, with the hazard ratio of 0.42.3
At the 18-month timepoint, the Kaplan-Meier estimates of progression-free survival were approximately 68% in the IMFINZI plus CP arm and approximately 32% in the CP alone arm. This analysis was exploratory and not designed to assess statistical significance. Another way to think of these data are that approximately 2 of 3 patients were estimated to be alive and progression free in the IMFINZI and CP arm at 18 months and approximately 1 of 3 patients in the CP alone arm.3
Post-hoc Exploratory Analysis of OS in the dMMR Subgroup
At the interim overall survival analysis, the median OS for the IMFINZI plus CP arm was not reached and was 23.7 months for the CP alone arm. At 18 months, about 86% of patients were estimated to be alive in the IMFINZI plus CP arm and about 66% in the CP alone arm. This post-hoc analysis was not designed to determine significance between the treatment arms. At this analysis, the OS data were immature, at 26%. Therefore, further follow up is needed.8
Post-hoc Exploratory Analysis of ORR in the dMMR Subgroup
In a post-hoc subgroup analysis of ORR in the dMMR subgroup, the objective response rate was approximately 71% in the IMFINZI plus CP arm and approximately 41% in the CP alone arm. Additionally, about 29% of patients achieved a complete response in the IMFINZI plus CP arm and around 10% achieved a complete response in the CP alone arm.9


X
Post-hoc Exploratory Analysis of DoR in the dMMR Subgroup
For those patients that responded, the median duration of response was not reached in the IMFINZI plus CP arm and was 10.5 months in the CP alone arm. At the 18-month timepoint, the percentage of patients remaining in response was about 75% in the IMFINZI plus CP arm and about 48% in the CP alone arm. Said differently, approximately 3 out of 4 patients that responded were still in response at 18 months in the IMFINZI plus CP arm.9
Overall, the addition of IMFINZI to CP led to a clinically meaningful improvement in PFS and an ORR around 71%. About 75% of patients treated with IMFINZI and CP who responded were estimated to remain in response at 18 months.3,9
Altogether, the DUO-E data add to the body of evidence that supports the use of immunotherapy plus CP followed by single agent immunotherapy for the treatment of primary advanced and recurrent dMMR endometrial cancer.1,6
Safety and Tolerability Profile for IMFINZI + CP in the dMMR Subgroup
While efficacy data are important to emphasize, it is also imperative to review safety and tolerability data of a treatment regimen.1
Safety data are available for a total of 44 patients with advanced or recurrent dMMR endometrial cancer who received IMFINZI with CP followed by IMFINZI as a single agent until disease progression or unacceptable toxicity.1
The median duration of exposure to IMFINZI with CP was 14.8 months, with a range of 0.7 to 31.7 in the dMMR subgroup.1
Serious adverse reactions occurred in 30% of patients who received IMFINZI with CP.1
The most common adverse reactions that occurred in more than 20% of patients (including laboratory abnormalities) were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, fatigue, abdominal pain, constipation, rash, decreased magnesium, increased ALT, increased AST, diarrhea, vomiting, cough, decreased potassium, dyspnea, headache, increased alkaline phosphatase, and decreased appetite.1


X
Clinically relevant adverse reactions in less than 10% of patients who received IMFINZI with CP included autoimmune hemolytic anemia, colitis, immune-mediated thyroiditis, infusion-related reaction, interstitial lung disease, myositis, pneumonitis, pulmonary embolism, and sepsis.1
Permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients.1
Dosage interruptions of IMFINZI due to adverse reactions occurred in 52% of patients.1
DUO-E Clinical Trial Key Points
Now that I’ve described the key DUO-E data supporting the FDA approval, I would like to leave you with a few key summary points.
The combination of IMFINZI and CP reduced the risk of progression or death by more than a half.3
Serious ARs occurred in 30% of patients receiving IMFINZI and CP. The five most common adverse reactions occurring in greater than 40% of patients were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, and fatigue.1
Importantly, the FDA approval of IMFINZI in combination with CP, followed by IMFINZI as a single agent, based on the DUO-E study, provides another option for patients in the treatment of primary advanced or recurrent dMMR endometrial cancer.1,6
Notably, it is important to test for mismatch repair status at diagnosis to ensure patients are eligible to receive precision medicine treatments.4
Continue to Important Safety Information >>>


X
References
1. IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
2. AstraZeneca. IMFINZI plus chemotherapy approved in the US for mismatch repair deficient advanced or recurrent endometrial cancer. Accessed March 2025. https://www.astrazeneca.com/media-centre/press-releases/2024/imfinzi-approved-in-the-us-for-endometrial-cancer.html
3. Westin SN, Moore K, Chon HS, et al; DUO-E Investigators. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283-299.
4. Hamoud BH, Sima RM, Vacaroiu IA, et al. The evolving landscape of immunotherapy in uterine cancer: A comprehensive review. Life (Basel). 2023;13(7):1502.
5. Cao SY, Fan Y, Zhang YF, Ruan JY, Mu Y, Li JK. Recurrence and survival of patients with stage III endometrial cancer after radical surgery followed by adjuvant chemo- or chemoradiotherapy: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):31.
6. Tillmanns T, Masri A, Stewart C, et al. Advanced endometrial cancer-The next generation of treatment: A society of gynecologic oncology journal club clinical commentary. Gynecol Oncol Rep. 2024;55:101462.
7. Data on File, REF-232005, AstraZeneca Pharmaceuticals LP; 2024.
8. Baurain J-F, Chon HS, Thomes-Pepin J, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab +/- olaparib as a first-line treatment for endometrial cancer: overall survival and additional secondary efficacy endpoints by mismatch repair status in the DUO-E/GOG-3041/ENGOT-EN10 trial. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
9. Chon HS, Thomes-Pepin J, Sundborg MJ, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab with or without olaparib as first-line treatment for endometrial cancer (DUO-E/GOG-3041/ENGOT-EN10): objective response rate and duration of response by mismatch repair status. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.

X
©2025 AstraZeneca. All rights reserved.
US-94253 Last Updated 5/25
References

Important Safety Information

Important Safety Information

Important Safety Information

Important Safety Information

IMPORTANT SAFETY INFORMATION
�There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal �immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.


X
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated.
Thyroid Disorders (Thyroiditis, Hyperthyroidism, and Hypothyroidism): IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.


X
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.


X
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.


X
Adverse Reactions
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), the most common adverse reactions, including laboratory abnormalities (occurring in ≥20% of patients) were peripheral neuropathy (61%), musculoskeletal pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%). The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
Please see Full Prescribing Information including Medication Guide for IMFINZI at IMFINZIhcp.com.
You are encouraged to report side effects related to AstraZeneca products by calling 1-800-236-9933. If you prefer to report these to the FDA, please call 1-800-FDA-1088.
Continue to References >>>


X
Transcript
Introduction
[Dr Westin] Hello! Thank you for joining us today. I’m Dr Shannon Westin, Professor and Clinical Medical Director in the Department of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center.
[Dr Backes] And I’m Dr Floor Backes, Professor in the Division of Gynecologic Oncology and Director of Gynecologic Cancer Research at The Ohio State University.
Today we will be discussing the aspects of the DUO-E trial design and our perspectives on how they may influence implementation into �clinical practice.
[Dr Westin] Before we proceed, we need to note that the opinions expressed in this video are solely our own and do not reflect �those of AstraZeneca.
Endometrial Cancer Prevalence
[Dr Backes] Now, let’s begin by highlighting the rising incidence of endometrial cancer and the shift in the treatment landscape.
Endometrial cancer is the most common gynecologic cancer and the fourth most common cancer overall in women in the United States.1,2
Endometrial Cancer Incidence
[Dr Backes] In the United States, it’s estimated that over 67,000 new cases of endometrial cancer may arise and over 13,000 deaths may �occur in 2024.3
Furthermore, the rate of new cases and death rates have been increasing over time. Specifically, incidence rates have continued to increase by 1% per year in White women and 2 to 3% in women of all other racial and ethnic groups. Furthermore, the death rate has risen by 1.7% per year.2

X
The rising incidence of endometrial cancer may be associated with several risk factors, including obesity, diabetes, changes in hormone �therapy use.4,5
Endometrial Cancer Survival Rates and Unmet Need
[Dr Backes] Most patients diagnosed with endometrial cancer are diagnosed with localized disease, with 5-year survival rates around 95%. However, those diagnosed with distant or metastatic disease tend to have poorer outcomes, with 5-year survival rates below 20%.3
This difference between survival rates in localized and distant stages highlights the unmet need for effective treatment options for patients with advanced or recurrent endometrial cancer.3,6
Regarding that treatment, the historical standard of care for advanced or recurrent endometrial cancer has been platinum-based chemotherapy with carboplatin plus paclitaxel regardless of biomarker status.7
However, the treatment landscape has evolved, with multiple combinations of immunotherapy plus chemotherapy now approved to treat patients with primary advanced or recurrent mismatch repair deficient endometrial cancer.7-9
DUO-E Study Overview
[Dr Westin] The underlying unmet medical need we just discussed led to the design of the DUO-E study. DUO-E is the first global, randomized, double-blind, placebo-controlled Phase 3 trial to evaluate IMFINZI in combination with first-line carboplatin and paclitaxel, or CP, followed by IMFINZI alone or an investigational combination regimen for patients with newly diagnosed advanced or recurrent endometrial cancer.7
While DUO-E investigated 3 arms, the current FDA approval is based on a prespecified dMMR subgroup, of 95 patients, in an exploratory analysis based on data from 2 arms, the control arm and the IMFINZI arm.8


X
DUO-E Study Design
[Dr Westin] Patients were recruited to the trial based on specific eligibility criteria, including but not limited to the following characteristics7:
All patients had newly diagnosed stage 3 or 4, or recurrent, endometrial cancer
Patients could have received adjuvant chemotherapy, but only if it has been at least 12 months from the last treatment to relapse
Patients with almost all histologies were included, including carcinosarcomas, but excluding sarcomas
Overall, 718 patients were randomized into the trial and were stratified based on mismatch repair status, geographical region, and disease status. 143 patients were randomized to the mismatch repair deficient, or dMMR, subgroup as part of a prespecified, exploratory analysis. Notably, stratification by MMR status was important for this trial, as dMMR tumors are more likely to produce abnormal proteins, or neoantigens, which may make them recognizable and susceptible to immunotherapy.7,10
Treatment during the trial can be separated into an induction phase and a maintenance phase, with the treatment schedules and doses shown. Note that IMFINZI or placebo is given every 4 weeks as a single agent in the maintenance phase compared to every 3 weeks in the induction phase with CP, with adjustments in dosing according to the time frame. Patients were treated until disease progression, unacceptable toxicity, or meeting other discontinuation criteria.7
The select primary endpoint of the study was progression-free survival, or PFS, in the intent-to-treat, or ITT population.8
Secondary endpoints included overall survival, overall response rate, and duration of response in the ITT population.8
Exploratory endpoints included a prespecified subgroup analysis of PFS by mismatch repair status, and post-hoc subgroup analyses of overall survival, overall response rate, and duration of response, by mismatch repair status.7,11,12


X
[Dr Backes] Thanks, Dr Westin. Overall, enrolling patients from many study locations around the world leads to an investigation in a diverse group of patients that may be representative of the real-world patient population. So, in my opinion, it’s critical for clinical trials to determine if an investigational treatment demonstrates safety and efficacy in diverse populations to help determine if the findings are generalizable to the patient population of patients at risk around the world.15
[Dr Westin] That’s a really good point to keep in mind for any clinical trial. When treating endometrial cancer with CP and immunotherapy regimens, how do you think geographic location may influence patient characteristics and treatment outcomes?
[Dr Backes] Well, we know that geographic location may be associated with factors like genetic alterations that can influence the diversity of the patient population and how a patient responds to treatment. Furthermore, genetic alterations associated with cancer development can be affected by many factors associated with geography, including ancestry, the environment, and lifestyle.16
And additionally, it’s important to note that different geographic locations follow different treatment practices, which may impact patient outcomes. For example, common treatment approaches vary for locally advanced disease. In some Asian countries, such as Japan, the standard treatment for locally advanced disease is surgery, with or without adjuvant chemotherapy, whereas, in western Europe and the United States, systemic therapy with or without radiation is more common.7,17
[Dr Westin] That makes sense and helps to highlight why it was important to stratify patients by geographic location, as was done in the DUO-E trial to help account for these variables.7
In my opinion, it’s important to look at geographic enrollment in a study and to consider how this may influence data interpretation.
Inclusion of Patients With Characteristics Associated With Aggressive Disease
[Dr Westin] Next, let’s discuss the inclusion of patients with characteristics associated with aggressive disease.7,14
In the dMMR subgroup, 37% of patients had newly diagnosed stage 4 advanced disease, 54% of patients had recurrent disease, 89% of patients had measurable disease at baseline, and 22% of patients had serous carcinoma, carcinosarcoma, mixed carcinoma, clear cell adenocarcinoma, and other histologies.14


X
Dr Backes, how do you think the patient characteristics in this trial compare to those of patients you see in your own practice? And are there any interesting characteristics you’d like to highlight?
[Dr Backes] Absolutely. These characteristics reflect what we typically encounter in our practice. One interesting characteristic is the inclusion of patients with carcinosarcomas, who represent a particularly challenging population to treat. Previously, these patients were excluded from trials because carcinosarcomas were considered a sarcoma, so they were overlooked in endometrial cancer clinical trials. Further, their rarity complicates the epidemiologic understanding of their nature.18
Endometrial carcinosarcoma cases are typically diagnosed at an advanced stage, display aggressive behavior, and are associated with a poor prognosis. Despite rising incidence rates and high recurrence rates of gynecologic carcinosarcomas, the optimal treatment has not been well established. Therefore, there is a need to investigate treatment regimens in these patients.18,19
[Dr Westin] All great points to keep in mind.
Within this study, it’s important to note that we cannot draw specific conclusions about how this treatment affects patients with
carcinosarcomas specifically, as the number of patients enrolled with this histology type is too small. Within the dMMR subgroup, �about 5% of patients had carcinosarcomas.14
However, we know from our practice that patients with aggressive disease are generally difficult to treat.
Overall, it was important to include patients with characteristics associated with aggressive disease in the DUO-E study, as this could increase the likelihood that findings might be relevant for these patients in real world clinical practice.
Chemotherapy-free Interval for Patients With Recurrent Endometrial Cancer
[Dr Westin] Now, let’s move on to discuss another criterion for trial eligibility in the DUO-E study. For patients with recurrent disease, prior adjuvant chemotherapy was allowed if at least 12 months had passed from last treatment to relapse. This represented approximately 12% of patients in the dMMR subgroup of the DUO-E study.7,14


X
IMPORTANT SAFETY INFORMATION
�There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal �immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.


X
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated.
Thyroid Disorders (Thyroiditis, Hyperthyroidism, and Hypothyroidism): IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.


X
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.


X
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.


X
Adverse Reactions
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), the most common adverse reactions, including laboratory abnormalities (occurring in ≥20% of patients) were peripheral neuropathy (61%), musculoskeletal pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%). The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
Please see Full Prescribing Information including Medication Guide for IMFINZI at IMFINZIhcp.com.
You are encouraged to report side effects related to AstraZeneca products by calling 1-800-236-9933. If you prefer to report these to the FDA, please call 1-800-FDA-1088.
Continue to References >>>


X
References
1. National Cancer Institute (NCI). Endometrial Cancer Treatment (PDQ®)–Health Professional Version. Accessed March 2025. https://www.cancer.gov/types/uterine/hp/endometrial-treatment-pdq#cit/section_1.1
2. American Cancer Society (ACS). Cancer Facts & Figures. 2024. Accessed March 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf
3. National Cancer Institute (NCI). Cancer Stat Facts: Uterine Cancer. Accessed March 2025. https://seer.cancer.gov/statfacts/html/corp.html
4. Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women's Health Initiative: An assessment of risk factors. J Womens Health (Larchmt). 2019;28(2):237-243.
5. Hamoud BH, Sima RM, Vacaroiu IA, et al. The evolving landscape of immunotherapy in uterine cancer: A comprehensive review. Life (Basel). 2023;13(7):1502.
6. Monk BJ, Smith G, Lima J, et al. Real-world outcomes in patients with advanced endometrial cancer: a retrospective cohort study of US electronic health records. Gynecol Oncol. 2022;164(2):325-332.
7. Westin SN, Moore K, Chon HS, et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283-299.
8. IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
9. Tillmanns T, Masri A, Stewart C, et al. Advanced endometrial cancer-The next generation of treatment: A society of gynecologic oncology journal club clinical commentary. Gynecol Oncol Rep. 2024;55:101462.
10. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54.
11. Baurain J-F, Chon HS, Thomes-Pepin J, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab +/- olaparib as a first-line treatment for endometrial cancer: overall survival and additional secondary efficacy endpoints by mismatch repair status in the DUO-E/GOG-3041/ENGOT-EN10 trial. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
12. Chon HS, Thomes-Pepin J, Sundborg MJ, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab with or without olaparib as first-line treatment for endometrial cancer (DUO-E/GOG-3041/ENGOT-EN10): objective response rate and duration of response by mismatch repair status. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.

X
Dr Backes, what do you think of this inclusion criterion? How might a 12-month timeframe between the last chemotherapy treatment and disease recurrence influence data interpretation?
[Dr Backes] Well, in my opinion, patients with disease recurrence soon after chemotherapy generally have a poorer prognosis compared to those with more time between prior treatment and disease recurrence. The influence of platinum-free intervals on patient outcomes has been investigated and validated in ovarian cancer, but there is a lack of comparable studies in endometrial cancer. So, there is definitely a need to further investigate platinum sensitivity in endometrial cancer to address this question. Additionally, the sample size of patients that had prior chemotherapy in the dMMR subgroup of the DUO-E study is too small to draw any conclusions based on this data alone.20-22
[Dr Westin] Great points. It is clear that more research in endometrial cancer is needed to determine if this patient characteristic is an important consideration, and if so, how it may influence response to another treatment.21,22
Treatment Until Disease Progression or Unacceptable Toxicity
[Dr Westin] Next, let’s discuss the fact that the clinical trial design continued treatment until disease progression or unacceptable toxicity. More specifically, during the induction phase, patients received IMFINZI and CP treatment every 3 weeks for a maximum of 6 cycles. After chemotherapy treatment concluded, patients were treated with IMFINZI alone every 4 weeks as maintenance treatment until disease progression or unacceptable toxicity. The IMFINZI dose during the induction phase was 1120 mg, followed by a dose of 1500 mg during the maintenance phase (unless the patient weighed less than 30 kg).7,8
[Dr Backes] In your opinion, Dr Westin, why could a maintenance dosing regimen until progression be a valuable clinical trial design feature?
[Dr Westin] I believe that having a maintenance dosing regimen until progression or unacceptable toxicity provides the opportunity to collect efficacy and safety data in an organized manner to ensure important data are not missed after a specific cutoff time. What do you think of this trial design decision?
[Dr Backes] I think having a study designed to treat patients until progression or unacceptable toxicity provides a valuable contribution to the endometrial cancer treatment data landscape.


X
[Dr Westin] While we’re discussing treatment duration, I’d also like to note that when reviewing clinical trial data, it’s helpful to check the median duration of treatment and exposure, as well as the data maturity. The medians help offer a sense of the real-world impact of treating until progression or unacceptable toxicity and the data maturity provides context for how likely it is that information could change at the next data analyses.
For the dMMR subgroup in the DUO-E trial, we know that median duration of exposure to IMFINZI with CP was 14.8 months (with a range of �0.7 to 31.7 months).8
It will be interesting to see how the median duration of exposure and treatment changes at future DUO-E data analyses as the data �continue to mature.
Statistical Analysis Plan
[Dr Westin] We’ve covered a lot, but let’s look at the final trial design feature we’d like to discuss today: the prespecified statistical analysis plan. The DUO-E trial was designed to assess progression-free survival in the intent-to-treat population for its primary endpoint. Investigators planned a prespecified exploratory subgroup analysis by mismatch repair status which was not designed to determine statistical significance between arms. However, the FDA agreed results from the dMMR subgroup analysis were clinically meaningful and thus granted AstraZeneca an approval for IMFINZI in combination with CP followed by IMFINZI as a single agent for the treatment of adult patients with primary advanced or recurrent dMMR endometrial cancer.7,8
Dr Backes, how confident are you in the data that support the IMFINZI FDA-approved indication?
[Dr Backes] Yes, the FDA approval provides support that the DUO-E data in the dMMR subgroup is clinically meaningful. I think this instills confidence to consider the IMFINZI regimen as another treatment option for patients with primary advanced or recurrent mismatch repair deficient endometrial cancer.
[Dr Westin] I agree.


X
Endometrial Cancer Treatment Landscape
[Dr Westin] Dr Backes and I covered a lot of information in a short amount of time, so we’ll use this opportunity to provide some key takeaways.
Following the approval of chemotherapy in the early 2000s to treat advanced or recurrent endometrial cancer, there was a lull in approvals. In 2023 and 2024, the treatment options expanded to incorporate immunotherapy and chemotherapy combination regimens as additional options to treat advanced or recurrent endometrial cancer, with IMFINZI and CP being approved in 2024 for the treatment of primary advanced or recurrent dMMR endometrial cancer.9,23
DUO-E Trial Design Features Conclusion
[Dr Westin] When evaluating these data, it’s important to remember that there are many trial design nuances that may influence implementation into clinical practice.
Several features discussed today include geographic location diversity, inclusion of characteristics associated with aggressive disease, the chemotherapy-free interval for patients with recurrent endometrial cancer, treatment until progression or unacceptable toxicity, and the statistical analysis plan.7,14
Dr Backes, are there any other key takeaways that you would like to add today?
DUO-E Trial Design Key Points
[Dr Backes] Yes, I have a few. The DUO-E study was designed to be representative of a diverse patient population, with trial sites around the world, and included patients with aggressive disease characteristics. These inclusive features are important to consider when implementing data into clinical practice.7
Overall, the DUO-E data add to the body of evidence that supports immunotherapy plus chemotherapy as a treatment option for primary advanced or recurrent endometrial cancer that is mismatch repair deficient. Also, testing patients for mismatch repair deficiency as soon as possible at diagnosis will be critical to ensure patients can access precision medicine treatments.5,8,9


X
[Dr Westin] That’s true. I think the most important takeaway to end with is that the IMFINZI plus chemotherapy combination indication offers an additional treatment option to treat patients with primary advanced or recurrent dMMR endometrial cancer.8,9
Continue to Important Safety Information >>>


X
References
13. Cao SY, Fan Y, Zhang YF, Ruan JY, Mu Y, Li JK. Recurrence and survival of patients with stage III endometrial cancer after radical surgery followed by adjuvant chemo- or chemoradiotherapy: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):31.
14. Data on File, REF-232005, AstraZeneca Pharmaceuticals LP; 2024.
15. Clougherty JE, Kinnee EJ, Cardet JC, et al. Geography, generalisability, and susceptibility in clinical trials. Lancet Respir Med. �2021;9(4):330-332.
16. American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed March 2025. https://cancerprogressreport.aacr.org/disparities/
17. Koppikar S, Oaknin A, Babu KG, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with endometrial cancer. ESMO Open. 2023;8(1):100774.
18. Bogani G, Ray-Coquard I, Concin N, et al. Endometrial carcinosarcoma. Int J Gynecol Cancer. 2023;33(2):147-174.
19. Lee JW, Ouh YT, Chang HK, et al. Trends in gynecologic carcinosarcoma based on analysis of the Surveillance Epidemiology End Result �(SEER) database. J Clin Med. 2023;12(3):1188.
20. Tomao F, D'Incalci M, Biagioli E, Peccatori FA, Colombo N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the �platinum-free interval: Myth or reality? Cancer. 2017;123(18):3450-3459.
21. Rubinstein M, Shen S, Monk BJ, et al. Looking beyond carboplatin and paclitaxel for the treatment of advanced/recurrent endometrial cancer. Gynecol Oncol. 2022;167(3):540-546.
22. Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258-279.
23. National Cancer Institute (NCI). More immunotherapy options approved for treating endometrial cancer. Accessed March 2025. https://www.cancer.gov/news-events/cancer-currents-blog/2024/endometrial-cancer-durvalumab-pembrolizumab-chemotherapy
©2025 AstraZeneca. All rights reserved.
US-94254 Last Updated 5/25

X

Transcript
Introduction
[Dr Backes] Hello! Thank you for joining us today. I’m Floor Backes, Professor in the Division of Gynecologic Oncology and Director of Gynecologic Cancer Research at The Ohio State University.
[Dr Westin] And I’m Dr Shannon Westin, Professor and Clinical Medical Director in the Department of Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center.
[Dr Westin] Today we will discuss the US indication for IMFINZI plus CP in patients with primary advanced or recurrent dMMR endometrial cancer, safety data, and physician insights on monitoring and managing select adverse events.
[Dr Backes] Before we proceed though, please note that the opinions expressed in this video are solely our own and do not reflect those �of AstraZeneca.
IMFINZI Endometrial Cancer Indication and Clinical Data
[Dr Westin] In June 2024, AstraZeneca received an FDA approval for IMFINZI in combination with carboplatin and paclitaxel followed by single-agent IMFINZI for the treatment of adult patients with primary advanced or recurrent dMMR endometrial cancer.1,2
This approval was based on the prespecified exploratory analysis of progression-free survival in the 95 patients in the dMMR subgroup from the DUO-E trial. IMFINZI plus CP led to a 58% reduction in the risk of progression or death. The median PFS was not reached for patients treated with IMFINZI and CP and 7.0 months for patients treated with CP, with a hazard ratio of 0.42.1,3
DUO-E Study Design
[Dr Westin] The DUO-E study was a global, randomized, double-blind, placebo-controlled, Phase III study of 718 patients with advanced or recurrent endometrial cancer.3

X
Patients with recurrent disease were required to have at least a 12-month chemotherapy-free interval to the date of their subsequent relapse with chemotherapy being delivered in the adjuvant setting. All histologies were eligible including carcinosarcomas, except sarcomas.3
Within the dMMR subgroup, 95 patients received first-line treatment with CP plus IMFINZI (or placebo) at 1120 mg once every three weeks for 6 cycles, followed by maintenance IMFINZI (or placebo) at 1500 mg once every four weeks. All treatments were given until disease progression or unacceptable toxicity. Note that the IMFINZI dose differs for patients lighter than 30 kg.1,3
One interesting aspect of this design is the relatively short dosing schedule, which brings patients to clinic more often. In my opinion, while patients may prefer to visit the clinic less, these visits provide more opportunities for patients to discuss any concerns with their physician to potentially detect or monitor any AEs to enable early interventions. It is important to ensure patients are counseled on possible AEs that may arise so they can report any concerns immediately.
Safety Data in the dMMR Subgroup
[Dr Westin] As we know, when determining a treatment plan for a patient, it is important to consider the safety profile of a treatment regimen and any adverse events that may occur. Now, let’s take a look at the safety profile of IMFINZI plus CP in the DUO-E trial.1
Safety data are available for a total of 44 patients with advanced or recurrent dMMR endometrial cancer who received IMFINZI with CP followed by IMFINZI as a single agent until disease progression or unacceptable toxicity. The median duration of exposure to IMFINZI with CP was 14.8 months, with a range of 0.7 to 31.7 months, in the dMMR subgroup.1
Serious adverse reactions occurred in 30% of patients who received IMFINZI with CP.1
Permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients.1
Dosage interruptions of IMFINZI due to adverse reactions occurred in 52% of patients.1


X
Clinically relevant adverse reactions in less than 10% of patients who received IMFINZI with CP included1:
Autoimmune hemolytic anemia
Colitis
Immune-mediated thyroiditis
Infusion-related reaction
Interstitial lung disease
Myositis
Pneumonitis
Pulmonary embolism and
Sepsis
[Dr Backes] The most common adverse reactions that occurred in more than 20% of patients, including laboratory abnormalities, were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, fatigue, abdominal pain, constipation, rash, decreased magnesium, increased ALT, increased AST, diarrhea, vomiting, cough, decreased potassium, dyspnea, headache, increased alkaline phosphatase, and decreased appetite.1
IMFINZI Dosage Modifications for Adverse Reactions
[Dr Backes] When we think about adverse reactions that may arise, it’s important to emphasize that patients and caregivers need counseling on signs and symptoms to look out for so they can detect and quickly report any possible AE. Ideally, it’s best to detect, and monitor, and manage any Grade 1 AE before it can progress to a higher grade, where it can become more challenging to mitigate.1
Note that AEs can happen during or after treatment, underscoring the importance of educating patients and caregivers to look out for new or worsening symptoms.1


X
If AEs are detected, dose reductions for IMFINZI are not recommended. In general, for severe Grade 3 immune-mediated adverse reactions, IMFINZI should be withheld. For any immune-mediated, life-threatening Grade 4 or recurrent severe Grade 3 immune-mediated adverse reactions that require systemic immunotherapy treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroid, in that case IMFINZI should be permanently discontinued. It’s important to consider the recommended dosage modifications for specific adverse reactions noted in the IMFINZI prescribing information (which is shown here).1
Immune-Mediated Adverse Reactions
[Dr Westin] Now that we’ve covered the key DUO-E safety data in the dMMR subgroup, we would like to discuss immune-mediated adverse reactions that may be relevant for this patient population.
IMFINZI is an immune checkpoint inhibitor that blocks the PD-1/PD-L1 pathway and removes inhibition of the immune response, which potentially breaks peripheral tolerance and may induce immune-mediated adverse reactions.1
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. These may include immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated dermatologic adverse reactions, immune-mediated nephritis and renal dysfunction, solid organ transplant rejection, and immune-mediated pancreatitis.1
[Dr Backes] Overall, identifying and managing immune-mediated adverse reactions is essential to help ensure the safe use of IMFINZI. The healthcare team should monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions.1
In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy with 1 to 2 mg/kg/day prednisone or equivalent until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid treatment.1


X
Patients should be encouraged to report any new symptoms quickly so clinicians can evaluate and decide on a course of action, which may include monitoring, a workup to exclude any alternative conditions, medical management, or evaluating whether IMFINZI may need to be withheld or discontinued.1
In addition, it’s important to evaluate the patient’s liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment with IMFINZI.1
[Dr Westin] Now that we’ve described the monitoring and management strategies for immune-mediated AEs, let’s discuss 3 specific AEs further. Specifically, we would like to focus on dermatitis or rash, thyroid-related adverse reactions, and pneumonitis.
Immune-Mediated Rash
[Dr Westin] First, let’s discuss immune-mediated rash or dermatitis. We thought it would be valuable to discuss this AE, as rash is one of the most common serious adverse reactions, with 4.5% of patients in the clinical trial experiencing the AE, and it also led to the highest percentage of permanent discontinuations of IMFINZI in 4.5% of patients who received IMFINZI with CP in the DUO-E trial.1
[Dr Backes] In addition, exfoliative dermatitis, including Stevens-Johnson Syndrome, drug rash with eosinophilia and systemic symptoms, or DRESS, and toxic epidermal necrolysis, or TEN, has occurred with PD-1/PD-L1 blocking antibodies.1
Usually what we would do is monitor skin-related symptoms such as rash, itching, skin blistering or peeling, painful sores or ulcers in the mouth or nose, throat, or genital area, fever or flu-like symptoms, or swollen lymph nodes.1
If mild to moderate non-exfoliative rashes arise, topical emollients and/or topical corticosteroids may be adequate to treat.1
Depending on the severity of the rash, the physician may need to withhold or permanently discontinue IMFINZI.1


X
Immune-Mediated Thyroid Disorders
[Dr Westin] The second group of AEs we’d like to discuss are immune-mediated thyroid disorders. For example, hypothyroidism can arise and has been reported in 11% of patients in the dMMR subgroup in DUO-E that received IMFINZI and CP.1
While this is not one of the most common AEs, we thought it would be interesting to discuss for this exact reason, as physicians may be less familiar with managing and monitoring thyroid-related AEs.
It is known that IMFINZI can cause immune-mediated thyroid disorders. Notably, thyroiditis can present with or without endocrinopathy, and hypothyroidism can follow hyperthyroidism. Therefore, it’s important to monitor thyroid function prior to and periodically during treatment.1
[Dr Backes] There are a number of endocrine symptoms to monitor that may be related to thyroid function issues, such as unusual headaches, exhaustion, changes in weight or appetite, and more.1
If hypothyroidism arises, hormone replacement therapy should be initiated. If hyperthyroidism arises, medical management should be initiated.1
Depending on the severity, IMFINZI should be withheld until the patient is clinically stable or should be permanently discontinued.1
Immune-Mediated Pneumonitis
[Dr Westin] The last immune-mediated AE we’d like to discuss is pneumonitis, which can be difficult to diagnose and can be serious.4
We know that IMFINZI can cause immune-mediated pneumonitis.1 In the DUO-E study, the presence of this AE required dosage interruptions of IMFINZI in 4.5% of patients who received IMFINZI with CP.1
[Dr Backes] In my practice, my course of action is to evaluate and monitor pulmonary symptoms including cough, shortness of breath, �and chest pain.1


X
If Grade 2 or higher pneumonitis is detected, I would withhold IMFINZI treatment. I’d resume IMFINZI in patients with complete or partial resolution (or Grade 0 to 1) after corticosteroid taper. If there was no complete or partial resolution within 12 weeks of initiating corticosteroids or an inability to reduce corticosteroid dose to 10 mg of prednisone or less per day (or equivalent) within 12 weeks of initiating corticosteroids, I’d permanently discontinue IMFINZI.1
If Grade 3 or 4 pneumonitis is detected, then I would permanently discontinue IMFINZI.1
[Dr Backes] As a reminder, the CTCAE provides unique clinical descriptions of the severity for each AE based on general guidelines. You can see the importance of identifying and treating pneumonitis as early as possible before symptoms become severe. It can be difficult to reverse pneumonitis once it progresses and the consequences are serious.6
DUO-E Safety Key Points
[Dr Westin] On behalf of Dr Backes and myself, I would like to thank you for tuning in today. I would like to leave you with a few key points to summarize this discussion.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue.1
Early identification of immune-mediated adverse reactions is essential to ensure safe use of IMFINZI.1
It is important to monitor for AEs during and following treatment and have a grade-specific plan in place to help manage AEs if they arise.7
Continue to Important Safety Information >>>


X
IMPORTANT SAFETY INFORMATION
�There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal �immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.


X
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated.
Thyroid Disorders (Thyroiditis, Hyperthyroidism, and Hypothyroidism): IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.


X
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.


X
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.


X
Adverse Reactions
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), the most common adverse reactions, including laboratory abnormalities (occurring in ≥20% of patients) were peripheral neuropathy (61%), musculoskeletal pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%). The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
Please see Full Prescribing Information including Medication Guide for IMFINZI at IMFINZIhcp.com.
You are encouraged to report side effects related to AstraZeneca products by calling 1-800-236-9933. If you prefer to report these to the FDA, please call 1-800-FDA-1088.
Continue to References >>>


X
References
1. IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
2. AstraZeneca. IMFINZI plus chemotherapy approved in the US for mismatch repair deficient advanced or recurrent endometrial cancer. Accessed March 2025. https://www.astrazeneca.com/media-centre/press-releases/2024/imfinzi-approved-in-the-us-for-endometrial-cancer.html
3. Westin SN, Moore K, Chon HS, et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283-299.
4. Helber HA, Hada AL, Pio RB, de Moraes PHZ, Gomes DBD. Immunotherapy-induced pneumonitis: cases report. Einstein (Sao Paulo). 2018;16(2):eRC4030.
5. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073-4126.
6. National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Updated November 27, 2017. Accessed March 2025. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
7. Sanchez K, Page DB, Urba W. Immunotherapy toxicities. Surg Oncol Clin N Am. 2019;28(3):387-401.
©2025 AstraZeneca. All rights reserved.
US-93745 Last Updated 5/25

X
Transcript
Introduction
Hello! Thank you for joining me today. I’m Dr Shannon Westin, Professor and Clinical Medical Director in the Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center.
And today we will discuss the US indication for IMFINZI in combination with carboplatin and paclitaxel in primary advanced or recurrent dMMR endometrial cancer and the data from the DUO-E trial that supported the FDA approval.
Before we proceed, I need to note that the opinions expressed in this video are solely my own and do not reflect those of AstraZeneca.
IMFINZI Endometrial Cancer Indication and Clinical Data
In June 2024, AstraZeneca received FDA approval for IMFINZI in combination with carboplatin and paclitaxel, known as CP, followed by IMFINZI as a single agent for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient, also known as dMMR.1,2
This approval was based on the prespecified exploratory analysis of progression-free survival in the dMMR subgroup that included 95 patients from the DUO-E trial. IMFINZI plus CP led to a 58% reduction in the risk of progression or death, with a hazard ratio of 0.42. The median progression-free survival was not reached in the IMFINZI plus CP arm and was 7 months in the CP arm.1,3
The IMFINZI-based regimen offers an additional treatment option to address a significant need for patients with advanced or recurrent endometrial cancer who have a five-year survival rate below 20%.1,4,5
The DUO-E data add to the body of evidence that support the use of immunotherapy plus CP for patients with endometrial cancer that is mismatch repair deficient. This underscores the importance of determining the mismatch repair status of the patient at diagnosis to offer a precision medicine approach to their treatment.1,4,6

X
DUO-E Study Design
The DUO-E study was a global, randomized, double-blind, placebo-controlled, Phase III study of 718 patients with advanced or recurrent endometrial cancer.3
Patients with recurrent disease were required to have at least a 12-month chemotherapy-free interval to the date of their subsequent relapse with chemotherapy being delivered in the adjuvant setting. All histologies were eligible including carcinosarcomas, except sarcomas.3
Within the dMMR subgroup, 95 patients received first-line treatment with CP plus IMFINZI (or placebo) at 1120 mg once every three weeks for 6 cycles, followed by maintenance IMFINZI (or placebo) at 1500 mg once every four weeks. All treatments were given until disease progression or unacceptable toxicity.3
For more details on the DUO-E trial design and how certain aspects may impact interpretations, consider watching the video called “The DUO-E Study Design: Physician Perspectives on Key Features.”
Patient Characteristics in the dMMR Subgroup
As for the patient baseline characteristics, these were generally balanced across treatment arms and representative of patients with newly diagnosed advanced or recurrent endometrial cancer.3
The DUO-E study was designed to enroll a patient group that is representative of the global real-world population. It included trial sites in North America, Europe and Asia, and included patients with advanced and/or aggressive disease.3,7
In the IMFINZI arm within the dMMR subgroup, 30% of patients enrolled were from Asia, while 70% of patients were from other areas around the world, labelled as non-Asia. This highlights the environmental and genetic diversity of the patients enrolled within the DUO-E study.3,8


X
Patients in both arms had characteristics associated with aggressive disease7:
37% had newly diagnosed Stage IV endometrial cancer
54% of patients had recurrent endometrial cancer
89% of patients had measurable disease at baseline, and
22% of patients had serous carcinoma, carcinosarcoma, mixed carcinoma, clear cell adenocarcinoma, or other histologies
Prespecified Exploratory Analysis of PFS in the dMMR Subgroup
As noted previously, FDA approval was based on the prespecified dMMR subgroup analysis of the DUO-E Phase III trial.1,3
In the dMMR subgroup, IMFINZI in combination with CP followed by IMFINZI as a single agent reduced the risk of disease progression or death by 58%, with the hazard ratio of 0.42.3
At the 18-month timepoint, the Kaplan-Meier estimates of progression-free survival were approximately 68% in the IMFINZI plus CP arm and approximately 32% in the CP alone arm. This analysis was exploratory and not designed to assess statistical significance. Another way to think of these data are that approximately 2 of 3 patients were estimated to be alive and progression free in the IMFINZI and CP arm at 18 months and approximately 1 of 3 patients in the CP alone arm.3
Post-hoc Exploratory Analysis of OS in the dMMR Subgroup
At the interim overall survival analysis, the median OS for the IMFINZI plus CP arm was not reached and was 23.7 months for the CP alone arm. At 18 months, about 86% of patients were estimated to be alive in the IMFINZI plus CP arm and about 66% in the CP alone arm. This post-hoc analysis was not designed to determine significance between the treatment arms. At this analysis, the OS data were immature, at 26%. Therefore, further follow up is needed.8
Post-hoc Exploratory Analysis of ORR in the dMMR Subgroup
In a post-hoc subgroup analysis of ORR in the dMMR subgroup, the objective response rate was approximately 71% in the IMFINZI plus CP arm and approximately 41% in the CP alone arm. Additionally, about 29% of patients achieved a complete response in the IMFINZI plus CP arm and around 10% achieved a complete response in the CP alone arm.9


X
Post-hoc Exploratory Analysis of DoR in the dMMR Subgroup
For those patients that responded, the median duration of response was not reached in the IMFINZI plus CP arm and was 10.5 months in the CP alone arm. At the 18-month timepoint, the percentage of patients remaining in response was about 75% in the IMFINZI plus CP arm and about 48% in the CP alone arm. Said differently, approximately 3 out of 4 patients that responded were still in response at 18 months in the IMFINZI plus CP arm.9
Overall, the addition of IMFINZI to CP led to a clinically meaningful improvement in PFS and an ORR around 71%. About 75% of patients treated with IMFINZI and CP who responded were estimated to remain in response at 18 months.3,9
Altogether, the DUO-E data add to the body of evidence that supports the use of immunotherapy plus CP followed by single agent immunotherapy for the treatment of primary advanced and recurrent dMMR endometrial cancer.1,6
Safety and Tolerability Profile for IMFINZI + CP in the dMMR Subgroup
While efficacy data are important to emphasize, it is also imperative to review safety and tolerability data of a treatment regimen.1
Safety data are available for a total of 44 patients with advanced or recurrent dMMR endometrial cancer who received IMFINZI with CP followed by IMFINZI as a single agent until disease progression or unacceptable toxicity.1
The median duration of exposure to IMFINZI with CP was 14.8 months, with a range of 0.7 to 31.7 in the dMMR subgroup.1
Serious adverse reactions occurred in 30% of patients who received IMFINZI with CP.1
The most common adverse reactions that occurred in more than 20% of patients (including laboratory abnormalities) were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, fatigue, abdominal pain, constipation, rash, decreased magnesium, increased ALT, increased AST, diarrhea, vomiting, cough, decreased potassium, dyspnea, headache, increased alkaline phosphatase, and decreased appetite.1


X
Clinically relevant adverse reactions in less than 10% of patients who received IMFINZI with CP included autoimmune hemolytic anemia, colitis, immune-mediated thyroiditis, infusion-related reaction, interstitial lung disease, myositis, pneumonitis, pulmonary embolism, and sepsis.1
Permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients.1
Dosage interruptions of IMFINZI due to adverse reactions occurred in 52% of patients.1
DUO-E Clinical Trial Key Points
Now that I’ve described the key DUO-E data supporting the FDA approval, I would like to leave you with a few key summary points.
The combination of IMFINZI and CP reduced the risk of progression or death by more than a half.3
Serious ARs occurred in 30% of patients receiving IMFINZI and CP. The five most common adverse reactions occurring in greater than 40% of patients were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, and fatigue.1
Importantly, the FDA approval of IMFINZI in combination with CP, followed by IMFINZI as a single agent, based on the DUO-E study, provides another option for patients in the treatment of primary advanced or recurrent dMMR endometrial cancer.1,6
Notably, it is important to test for mismatch repair status at diagnosis to ensure patients are eligible to receive precision medicine treatments.4
Continue to Important Safety Information >>>


X
IMPORTANT SAFETY INFORMATION
�There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal �immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.


X
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated.
Thyroid Disorders (Thyroiditis, Hyperthyroidism, and Hypothyroidism): IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.


X
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.


X
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.


X
Adverse Reactions
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), the most common adverse reactions, including laboratory abnormalities (occurring in ≥20% of patients) were peripheral neuropathy (61%), musculoskeletal pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%). The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single-agent (n=44), permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
Please see Full Prescribing Information including Medication Guide for IMFINZI at IMFINZIhcp.com.
You are encouraged to report side effects related to AstraZeneca products by calling 1-800-236-9933. If you prefer to report these to the FDA, please call 1-800-FDA-1088.
Continue to References >>>


X
References
1. IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
2. AstraZeneca. IMFINZI plus chemotherapy approved in the US for mismatch repair deficient advanced or recurrent endometrial cancer. Accessed March 2025. https://www.astrazeneca.com/media-centre/press-releases/2024/imfinzi-approved-in-the-us-for-endometrial-cancer.html
3. Westin SN, Moore K, Chon HS, et al; DUO-E Investigators. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283-299.
4. Hamoud BH, Sima RM, Vacaroiu IA, et al. The evolving landscape of immunotherapy in uterine cancer: A comprehensive review. Life (Basel). 2023;13(7):1502.
5. Cao SY, Fan Y, Zhang YF, Ruan JY, Mu Y, Li JK. Recurrence and survival of patients with stage III endometrial cancer after radical surgery followed by adjuvant chemo- or chemoradiotherapy: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):31.
6. Tillmanns T, Masri A, Stewart C, et al. Advanced endometrial cancer-The next generation of treatment: A society of gynecologic oncology journal club clinical commentary. Gynecol Oncol Rep. 2024;55:101462.
7. Data on File, REF-232005, AstraZeneca Pharmaceuticals LP; 2024.
8. Baurain J-F, Chon HS, Thomes-Pepin J, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab +/- olaparib as a first-line treatment for endometrial cancer: overall survival and additional secondary efficacy endpoints by mismatch repair status in the DUO-E/GOG-3041/ENGOT-EN10 trial. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
9. Chon HS, Thomes-Pepin J, Sundborg MJ, et al. Durvalumab + carboplatin/paclitaxel followed by durvalumab with or without olaparib as first-line treatment for endometrial cancer (DUO-E/GOG-3041/ENGOT-EN10): objective response rate and duration of response by mismatch repair status. Presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
©2025 AstraZeneca. All rights reserved.
US-94253 Last Updated 5/25

X