







Designing medical devices that are secure
and comply with cyber security legislation,
legal requirements and regulatory guidance

Makers of connected medical devices, have seen an increasing need to implement more stringent cybersecurity measures to prevent hackers from taking control of medical devices or accessing confidential medical data. The US FDA, EU MDR and other medical device regulatory agencies have encouraged medical device makers to follow international security standards and to transition from software security methods to using hardware security elements.
Microchip’s hardware security solutions help your design to meet the government regulatory cybersecurity guidance and international security standards as well as to transition from software security methods to using hardware security elements.

For more information go to:

Microchip Security Solutions



Designing medical devices that are
properly integrated into a Cloud service

Choosing the right cloud service, connectivity model and security strategy for an Internet of Medical Things design is critical. Medical device makers have realized that they need to make this choice early in the design process to avoid schedule slips that can increase their development costs and
time to market.
Microchip simplifies the complex task of integrating the products and services that you need to connect your Internet of Medical Things designs. For example, we offer pre-certified development platforms for use with multiple cloud providers such as Amazon AWS and Microsoft Azure to help jump-start
your medical device designs.

For more information go to:

Microchip IoT Solutions



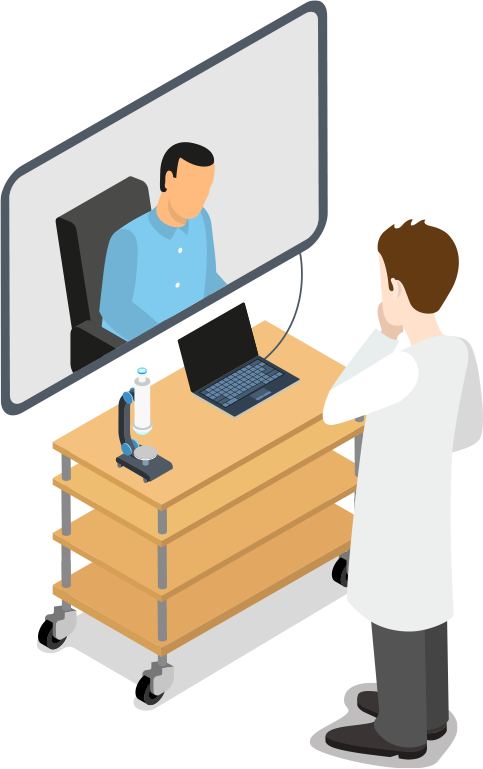
Designing Home Telehealth Devices

The demand for remote and in-home telehealth services has grown significantly. Hospitals, clinics, and other medical providers are requiring more telehealth device solutions to help limit the spread of infectious diseases, to expand coverage to medically underserved populations and to significantly reduce costs.
Telehealth device designers need an experienced and medical-focused semiconductor solutions supplier to help meet the demands of the current market. Microchip has a long reputation of helping our medical customers get to market faster with robust telehealth designs.

For more information go to:

Functional Safety Ecosystem and IEC-62304
Functional Safety
Medical and Advanced Packaging Services

Telehealth Reference Solutions
Wireless Connectivity
Sensor measurement and front-end processing




Designing Clinical Telehealth Devices

The demand for remote and in-home telehealth services has grown significantly. Hospitals, clinics, and other medical providers are requiring more telehealth device solutions to help limit the spread of infectious diseases, to expand coverage to medically underserved populations and to significantly reduce costs.
Telehealth device designers need an experienced and medical-focused semiconductor solutions supplier to help meet the demands of the current market. Microchip has a long reputation of helping our medical customers get to market faster with robust telehealth designs.

For more information go to:

Functional Safety Ecosystem and IEC-62304
Functional Safety
Medical and Advanced Packaging Services

Telehealth Reference Solutions
Wireless Connectivity
Sensor measurement and front-end processing



Designing Connected Home Drug
Delivery Devices

Many medications are starting to be administered at home by caregivers or by the patients themselves. This makes electronic patient compliance a necessity to accurately manage medication therapy and to offer legal protection for medical providers.
Microchip can help you create low-power, compact and connected drug delivery devices that lower the overall cost and risk often associated with the innovation and development of patient compliant medical solutions.

For more information go to:

Functional Safety Ecosystem and IEC-62304
Functional Safety
Medical and Advanced Packaging Services

Drug Delivery Reference Solutions
Authentication for accessories and disposables
Wireless Connectivity
Motor Control




Designing Connected Clinical Drug
Delivery Devices

Many medications are starting to be administered at home by caregivers or by the patients themselves. This makes electronic patient compliance a necessity to accurately manage medication therapy and to offer legal protection for medical providers.
Microchip can help you create low-power, compact and connected drug delivery devices that lower the overall cost and risk often associated with the innovation and development of patient compliant medical solutions.

For more information go to:

Functional Safety Ecosystem and IEC-62304
Functional Safety
Medical and Advanced Packaging Services

Drug Delivery Reference Solutions
Authentication for accessories and disposables
Wireless Connectivity
Motor Control




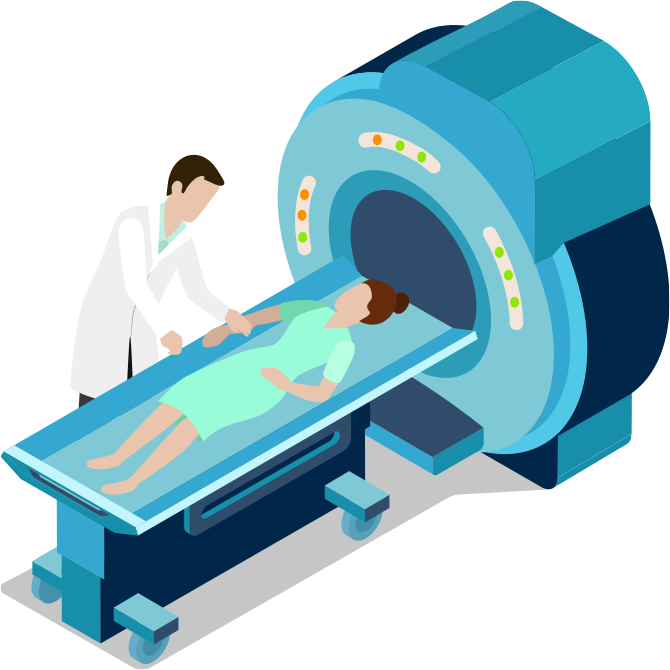
Designing Connected Imaging Devices

The healthcare industry is seeing a strong and a steady growth around the globe, resulting in an increased demand for non-invasive medical imaging modalities. Many of these have evolved over the years from large and heavy, stationary or trolley-based systems to lightweight and portable devices that are often handheld, and battery powered for use in Point-of-Care (POC) applications.
Partnering with Microchip on your next medical device design can help you decrease the complexity of your design process, reduce your development costs, and speed up your time to market.

For more information go to:

Wired Connectivity-USB
Functional Safety Ecosystem and IEC-62304
Functional Safety
32-bit Microprocessors/Operating Systems

FPGA Processing
Ultrasound high-voltage, front-end devices
Wireless Connectivity
Wired Connectivity-Ethernet



Designing Connected Clinical Devices

Hospitals and clinics require more complex medical devices that not only offer advanced diagnostic and therapeutic capabilities, but also must meet increasingly demanding requirements from government regulatory bodies and increasing demands of new global developing markets.
Microchip routinely engages in supporting our medical device clients even with the toughest designs, such as FDA Class III devices as well as helping them to meet ISO-13485, IEC-62304 and other international standards.

For more information go to:

Motor Control
Sensor measurement and front-end processing
Functional Safety Ecosystem and IEC-62304
Functional Safety
32-bit Microprocessors/Operating Systems

Clinical Reference Solutions
Wireless Connectivity
Wired Connectivity-Ethernet
Wired Connectivity-USB
FPGA Processing



Connected Hospitals/Clinics/Physicians' Offices


Connected Homes/Remote Locations






Designing medical devices that
are secure and comply with
cyber security legislation, legal
requirements and regulatory guidance


Designing medical devices
that are properly integrated
into a Cloud service


Designing Connected
Home Drug Delivery Devices


Designing Connected
Clinical Drug Delivery Devices


Designing Connected
Imaging Devices


Designing Connected
Clinical Devices


Designing Home
Telehealth Devices


Designing Clinical
Telehealth Devices












































For more information go to:
Microchip Security Solutions
How to Design Secure Medical Devices Based On the FDA’s Cybersecurity Guidance

For more information go to:
FPGA Processing
Ultrasound High-Voltage and Front-End Devices
Power Management With Silicon Carbide
32-bit Microprocessors/Operating Systems
Functional Safety Ecosystem and IEC 62304

For more information go to:
Clinical Reference Solutions
FPGA Processing
Power Management With Silicon Carbide
32-bit Microprocessors/Operating Systems
Motor Control
Sensor Measurement and Signal Chain
Functional Safety Ecosystem and IEC 62304
For more information go to:
Drug Delivery Reference Solutions
Authentication for Accessories and Disposables
Arm® Cortex®-Based 32-bit MCUs
8-bit PIC® and AVR® Microcontrollers
Motor Control
Functional Safety Ecosystem and IEC 62304
For more information go to:
Drug Delivery Reference Solutions
Authentication for Accessories and Disposables
Arm® Cortex®-Based 32-bit MCUs
8-bit PIC® and AVR® Microcontrollers
Motor Control
Functional Safety Ecosystem and IEC 62304
For more information go to:
Telehealth Reference Solutions
Arm® Cortex®-Based 32-bit MCUs
8-bit PIC® and AVR® Microcontrollers
Sensor Measurement and Signal Chain
Functional Safety Ecosystem and IEC 62304
For more information go to:
Telehealth Reference Solutions
Arm® Cortex®-Based 32-bit MCUs
8-bit PIC® and AVR® Microcontrollers
Sensor Measurement and Signal Chain
Functional Safety Ecosystem and IEC 62304
The healthcare industry is seeing a strong and a steady growth around the globe, resulting in an increased demand for non-invasive medical imaging systems. Many of these large and heavy stationary or trolley-based systems have evolved into lightweight and portable devices that are frequently handheld and battery powered for use in Point-of-Care (POC) applications.
We are experienced in supporting makers of medical imaging devices and have demonstrated that we can help decrease the complexity of the development process, reduce costs and speed up the time to market for these devices.
Designing Connected Imaging Devices
Hospitals and clinics require more complex medical devices that offer advanced diagnostic and therapeutic capabilities. These devices must be designed to meet the requirements of emerging global markets and government regulatory bodies.
We support our medical device clients with their toughest design challenges, including FDA Class III devices. We also help them meet ISO 13485, IEC 62304 and other international standards.
Designing Connected Clinical Devices
Many medications are starting to be administered at home by caregivers or by the patients themselves. This makes electronic patient compliance a necessity to accurately manage medication therapy and to offer legal protection for medical providers.
We can help you create low-power, compact and connected drug delivery devices that lower the overall cost and risk associated with the innovation and development of patient compliant medical solutions.
Designing Connected Home Drug Delivery Devices
Many medications are starting to be administered at home by caregivers or by the patients themselves. This makes electronic patient compliance a necessity to accurately manage medication therapy and to offer legal protection for medical providers.
We can help you create low-power, compact and connected drug delivery devices that lower the overall cost and risk associated with the innovation and development of patient compliant medical solutions.
Designing Connected Clinical Drug Delivery Devices
Hospitals, clinics and other medical providers are requiring more telehealth device solutions to help limit the spread of infectious diseases, to expand coverage to medically underserved populations and to significantly reduce costs.
Telehealth device designers need an experienced and medical-focused semiconductor solutions supplier to help meet the demands of the current market. We have a long history of helping our clients get to market faster with robust medical designs.
Designing Home Telehealth Devices
Hospitals, clinics and other medical providers are requiring more telehealth device solutions to help limit the spread of infectious diseases, to expand coverage to medically underserved populations and to significantly reduce costs.
Telehealth device designers need an experienced and medical-focused semiconductor solutions supplier to help meet the demands of the current market. We have a long history of helping our clients get to market faster with robust medical designs.
Designing Clinical Telehealth Devices
Makers of connected medical devices have seen an increasing need to implement more stringent cybersecurity measures to prevent hackers from taking control of medical devices or accessing confidential medical data. Medical device regulatory agencies, such as the US FDA, have urged medical device makers to follow international security standards. They have also encouraged them to transition from software security methods to using hardware security elements.
Designing Medical Devices That Are Secure and Comply With Cybersecurity Legislation, Legal Requirements and Regulatory Guidance

Our hardware security solutions help your designs meet commercial security requirements, government regulatory cybersecurity guidance and international security standards. We can also help you transition from software security methods to using hardware security elements.

Choosing the right cloud service, connectivity model and security strategy for an Internet of Medical Things design is critical. Medical device makers have realized that they need to make this choice early in the design process to avoid schedule slips that can increase their development costs and time to market.
We simplify the complex task of integrating the products and services that you need to connect your Internet of Medical Things designs by offering pre-certified development platforms for use with multiple cloud providers to help jump-start your medical device designs.
Designing Medical Devices That Are Properly Integrated With a Cloud Service


Designing Connected
Imaging Devices
Designing Connected
Clinical Devices
Designing Connected
Home Drug Delivery Devices
Designing Connected Clinical Drug Delivery Devices
Designing Clinical
Telehealth Devices




Makers of connected medical devices, have seen an increasing need to implement more stringent cybersecurity measures to prevent hackers from taking control of medical devices or accessing confidential medical data. Medical device regulatory agencies, such as the US FDA have encouraged medical device makers to follow international security standards and to transition from software security methods to using hardware security elements.
Microchip’s hardware security solutions help your designs meet commercial security requirements government regulatory cybersecurity guidance and international security standards as well as help you transition from software security methods to using hardware security elements.
Designing Medical Devices that are Secure and Comply with Cyber Security Legislation, Legal Requirements and Regulatory Guidance

For more information go to:
Explore AWS
Designing Medical Devices That Are Secure
and Comply With Cybersecurity Legislation,
Legal Requirements and Regulatory Guidance
Designing Home
Telehealth Devices
Designing Medical
Devices That Are Properly
Integrated With a Cloud Service









For more information go to:
Explore AWS
Connect to Microsoft Azure
Professional IoT Design Services